Peer Reviewed
Unmasking the Culprit of PVCs: Left Atrial Myxoma Diagnosed Preoperatively
A 46-year-old woman presented in an outpatient cardiology office for preoperative clearance for tubal ligation surgery. On assessment, the physicians found her to have frequent premature ventricular contractions (PVCs).
History. The patient’s past medical history was notably insignificant for cardiac pathologies or neoplastic processes. Her medical history was significant for depression, but no other significant personal history was noted. The only medication was citalopram 40 mg once daily, which the patient reported taking as prescribed.
Her familial medical history was significant for both parents having unspecified heart disease. Otherwise, the family history was unremarkable.
She presented with a regular heart rate with symmetrically palpable distal pulses in all four extremities. The examination revealed no gallop or friction rub and cardiac murmurs were not appreciated. Although the patient presented with an elevated respiratory rate of 28 breaths/min, she was not in respiratory distress.
Basic laboratory testing revealed a sodium level of 140 mEq/L, potassium level of 4.1 mEq/L, chloride level of 109 mmol/L, creatinine level of 0.65 mg/dL, and blood urea nitrogen of 12 mg/dL.
Diagnostic testing. The physical examination was largely unremarkable other than frequent premature beats noted on auscultations. The patient’s pre-cardiac catheterization electrocardiogram results, including the QTc, were within normal limits. Subsequent Holter monitor studies revealed 33 premature ventricular contractions (PVC) couplets and three short runs of nonsustained ventricular tachycardia. Although citalopram is a potential culprit of such findings, further testing was pursued to rule out other etiologies.
A stress test revealed reversible ischemia and subsequent heart catheterization performed as soon as possible revealed a vascularized cardiac mass fed by the first obtuse marginal artery (Figure 1). Further testing was done with transesophageal echocardiography (TEE) to better visualize the left atrial mass. TEE was conducted as opposed to transthoracic echocardiography based on the high level of clinical suspicion from the cardiac catheterization images. TEE visualized a 1.7 cm X 1.2 cm pedunculated mass in the left atrium connected to the interatrial septum (Figure 2).
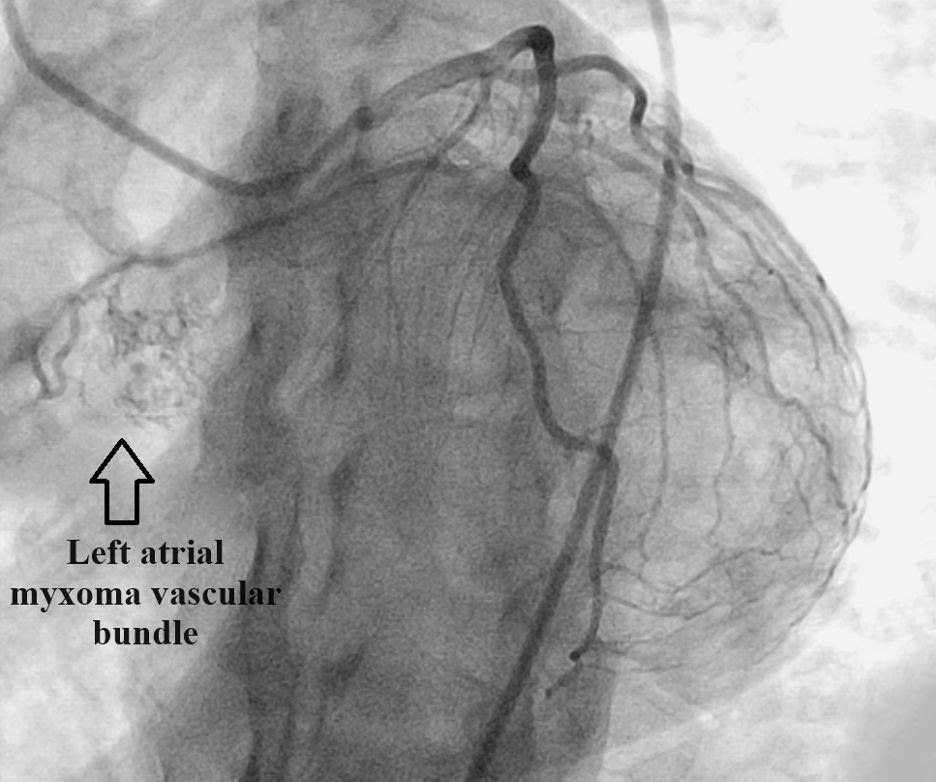
Figure 1. Heart catheterization revealing a highly vascularized left atrial mass fed by the first obtuse marginal artery.
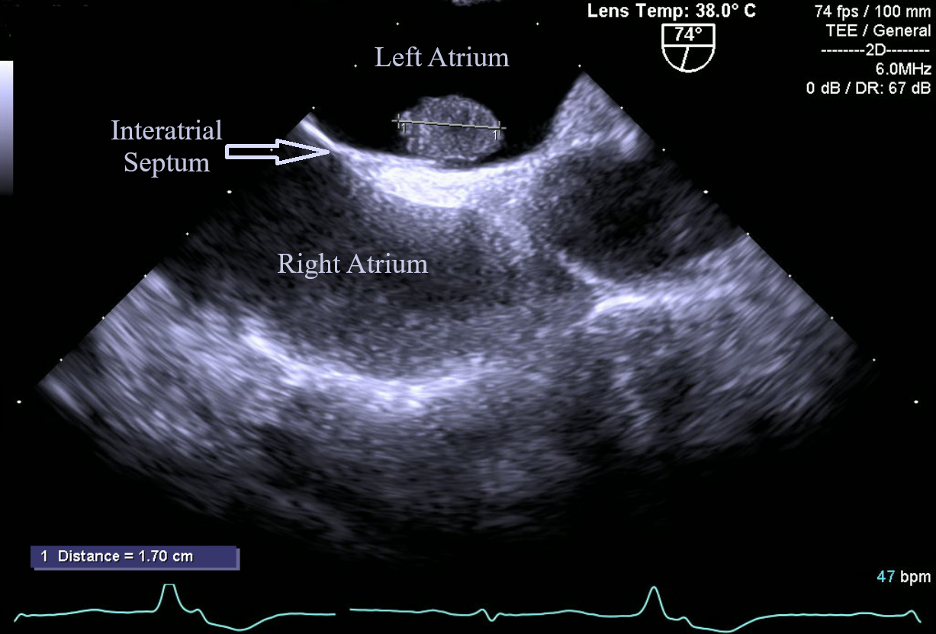
Figure 2. Left atrial myxoma (1.7 cm X 1.2 cm) visualized through TEE. This image depicts the classic shape and location of myxomas; a pedunculated mass arising from the interatrial septum.
Based on testing results and the benign appearance of the mass, all evidence pointed towards this mass being a benign atrial myxoma. Not only is atrial myxoma the most common benign heart mass, but this diagnosis was also deemed very likely based on the classic location and appearance of a left atrial pedunculated mass attached to the interatrial septum.
Differential diagnoses. Benign atrial myxoma was at the top of our differential after undergoing the previously mentioned diagnostic workup.
We considered the possibility that it was a secondary cardiac tumor via distal metastasis to the heart. However, the patient’s history and physical examination were not suggestive of malignancy. Furthermore, TEE showed a single, regular shape, pedunculated and, what appeared to be, a non-invasive mass. These findings were each suggestive that the mass was benign.
We also considered some other benign cardiac tumors. A papillary fibroelastoma was less likely because it is almost exclusively found on heart valves.1 Rhabdomyoma was also less likely as it is typically seen in pediatric cases.1 A fibroma was another consideration but is typically found within the left ventricle.1 Although a lipoma was in the differential, these are quite rare and are more often incorporated within the wall of the heart, rather than a unique discrete tumor within a chamber.1
Treatment and follow-up. Initially, the patient was started on metoprolol tartrate 25 mg twice daily, which was quickly increased to 50 mg twice daily in effort to suppress PVCs and nonsustained ventricular tachycardia. A repeat Holter monitor following the increase to 50 mg metoprolol twice daily revealed a moderate increase in the frequency of PVCs, but also a significant decrease in PVC couplets and no runs of nonsustained ventricular tachycardia.
Given the patient's age and potential risks associated with the tumor (including embolization and hemodynamic complications), surgical resection of the left atrial myxoma was recommended by the cardiothoracic surgeon. A standard median sternotomy was performed, and the chest was opened. Ultimately, cardiopulmonary bypass was initiated, which allowed for the surgeon to perform the procedure and remove the tumor in total. The surgery was successfully completed without any complications.
Outcome and follow-up. The patient’s frequent PVCs declined almost immediately post-operatively and declined to less than 2% of total beats 30 days post-operatively. The tumor was sent for pathology and confirmed the diagnosis of myxoma. The post-operative phase was uneventful with no recurrence of the neoplasm.
Discussion. Primary cardiac tumors, of which myxomas are the most common, have an incidence of around 0.02% in the general population.2 Meanwhile, studies indicate that secondary cardiac tumors are relatively common and 8% of cancer mortality cases have metastatic disease to the heart.3
Myxomas most commonly arise sporadically from primary connective tissue and have no clear etiology.4 The female-to-male ratio for diagnosis is 2:1 with most cases occurring in the 30 to 60 years of age range.4 Of all cardiac myxomas, approximately 80% of cases occur in the left atrium, most commonly arising from the interatrial septum.5 This location, along with the pedunculated shape, is indicative of myxomas.5
Classic signs and symptoms associated with myxomas are a classic low frequency heart sound heard in early diastole (known as a “tumor plop”), obstructive cardiac signs, embolism, a regurgitation murmur, ectopy, and constitutional symptoms.4 This case is unique in that the patient was asymptomatic and the only sign of an underlying issue was the finding of PVCs.
One significant complication of myxomas is the possibility of systemic embolization of the tumor, something that is present in about 29% of patients.6 This can lead to stroke, neurologic deficits, and death. Myxomas that are soft/friable and smaller in size (less than 4.5 cm) are at an increased risk of embolization.6
Conclusion. Despite the rarity of primary cardiac neoplasms, it is important for practitioners to consider this in their differential diagnosis even with seemingly benign asymptomatic PVCs, since treatment delays can have serious consequences. Recognizing potential presentations of asymptomatic atrial myxoma and conducting appropriate tests is vital for timely intervention and improved patient outcomes. This case serves as a stark reminder of the importance of thorough pre-operative assessments as workup can reveal potentially life-threatening conditions. Cardiac myxoma, although benign, is a cardiac neoplasm that must be quickly addressed since complications from this tumor can be fatal.
1. Casavecchia G, Lestuzzi C, Gravina M, et al. Cardiac tumors. J Cardiovasc Echogr. 2020;30(Suppl 1), S45–S53. doi:10.4103/jcecho.jcecho_7_19
2. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77(1):107. doi:10.1016/s0002-9149(97)89149-7
3. Silvestri F, Bussani R, Pavletic N, Mannone T. Metastases of the heart and pericardium. G Ital Cardiol. 1997;27(12):1252-1255.
4. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma: a series of 112 consecutive cases. Medicine. 2001;80(3):159-172 doi:10.1097/00005792-200105000-00002
5. Garatti A, Nano G, Canziani A, et al. Surgical excision of cardiac myxomas: twenty years experience at a single institution. Ann Thorac Surg. 2012;93(3):825-831. doi:10.1016/j.athoracsur.2011.11.009
6. Wang Z, Chen S, Zhu M, et al. Risk prediction for emboli and recurrence of primary cardiac myxomas after resection. J Cardiothorac Surg. 2016;11:22. doi:10.1186/s13019-016-0420-4
AFFILIATIONS:
1MercyHealth Tiffin Cardiology Specialists, Tiffin, OH
2Anesthesiology Resident, The Ohio State University, Columbus, OH
3Student, Siena Heights University, Adrian, MI
CITATION:
Bruhl SR, Slembarski AJ, Sluss GN. Unmasking the culprit of PVCs: left atrial myxoma diagnosed preoperatively. Consultant. 2024;64(5):e1. doi:10.25270/con.2024.05.000002
Received September 21, 2023. Accepted February 5, 2024. Published online May 14, 2024.
DISCLOSURES:
The authors report no relevant financial relationships.
ACKNOWLEDGEMENTS:
None.
CORRESPONDENCE:
Steven R. Bruhl, Mercy Health Tiffin Cardiology Specialists, 45 St Lawrence Drive, Tiffin, OH 44883 (steven_bruhl1@mercy.com)


