Peer Reviewed
A Rare Case Report on Radiation Recall Pneumonitis in a Patient Treated with Durvalumab for Non-Small Cell Lung Cancer
Introduction. A 77-year-old man first presented to the emergency department (ED) due to hemoptysis and intermittent shortness of breath. The patient was diagnosed with non-small cell lung cancer (NSCLC) and was treated by chemoradiation. The patient was then started on maintenance immunotherapy with a checkpoint inhibitor (received five cycles of Durvalumab) before he presented again 4 months later with hemoptysis and dyspnea.
History. The patient is a former smoker with a 52-pack-year smoking history who quit 20 years ago. His past medical history is significant for coronary artery disease (CAD), post coronary artery bypass grafting (CABG), atrial fibrillation, hypothyroidism, and gastroesophageal reflux disease (GERD).
Diagnostic testing. The patient was relatively stable on physical examination during his first visit to the ED, with good bilateral air entry and no wheezing. Laboratory results showed a white blood cell (WBC) count of 4.9 × 10³/µL (4.5 - 11.0 × 10³/µL), hemoglobin of 11.9 g/dL (14 - 18 g/dL), platelet count of 122 × 10³/µL (150 - 350 x 10³/µL), blood urea nitrogen (BUN) of 17 mg/dL (8 20 mg/dL), creatinine (Cr) of 1.13 mg/dL (0.8 - 1.3 mg/dL), and electrolyte levels of sodium (Na) 137 mEq/L (136 - 145 mEq/L ) and potassium (K) 4.3 mEq/L (3.5 - 5.0 mEq/L). Liver function tests (LFTs) were within normal limits. The initial workup included computed tomography angiography (CTA) of the chest, which revealed a right suprahilar mass measuring 3.5 × 2.6 cm (Figure 1), suspicious for malignancy.
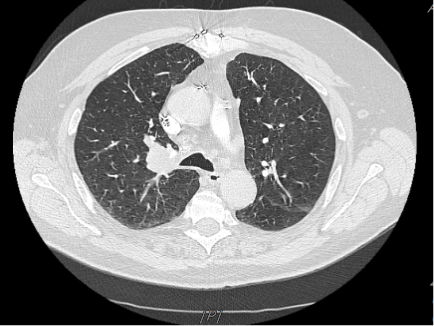
Figure 1. Chest CT at the diagnosis showed a right suprahilar mass.
Diagnostic fiberoptic bronchoscopy showed no active bleeding at the time. Endobronchial biopsies of the right upper lobe showed moderate to poorly differentiated squamous cell carcinoma. Positron emission tomography (PET)/computed tomography (CT) fusion from skull to thigh was done that showed hypermetabolic irregular 3.5 x 2.6 cm right upper lobe (RUL) mass consistent with underlying malignancy showing standardized uptake value (SUV) of 11 (> 2.5 suggestive of malignancy), along with paratracheal and subcarinal lymph node hypermetabolic uptake of 3.5-4.0. Mediastinal lymph node biopsy by endobronchial ultrasonography (EBUS) bronchoscopy was positive for metastasis. CT of the head showed no evidence of metastasis. Therefore, the patient was staged as unresectable stage IA NSCLC (squamous cell carcinoma) of the RUL of the lung with planned concomitant chemoradiation treatment followed by maintenance immunotherapy.
Differential diagnoses. Durvalumab is a PD-L1 inhibitor class of drugs, and it is known to cause immune-related adverse events (irAEs), one of which is checkpoint inhibitor pneumonitis (CIP). The pneumonitis can also result from radiation therapy alone (aka radiation pneumonitis [RP]), or radiation therapy followed by treatment with an immune checkpoint inhibitor (ICI) such as durvalumab, resulting in radiation recall pneumonitis (RRP). The differential diagnosis among CIP, RP, and RRP can be challenging as many diagnostic features overlap. The CT scans indicate that: (1) CIP causes bilateral organizing pneumonia, involving more lobes and less sharp borders1; (2) RP exhibits sharp borders with unilateral changes on the same side of the primary lung tumor2; (3) RRP show ground-glass opacity (GGO) or more focal consolidation in the previously irradiated field.3
Treatment and management. The patient underwent radiation therapy with a prescription dose of 66 Gy in 33 fractions. Chemotherapy was started with carboplatin and paclitaxel for 6 weeks. One month after treatment, a restaging workup showed a near-complete response, with the disappearance of the right hilar mass (Figure 2). The patient was then started on maintenance immunotherapy with checkpoint inhibitor Durvalumab. The dosage for durvalumab is calculated based on patient body weight. For non-small cell lung cancer, the dosage is 10 mg/kg IV for 60 minutes every 2 weeks or as a consultant oncologist deems necessary.

Figure 2. Chest CT, 1 month after finishing chemotherapy, showing near complete resolution of the mass.

Figure 3. Chest CT after five cycles of Durvalumab showing a reappearance of RUL consolidation with air bronchograms.
Outcome and follow-up. In this case, the patient initially presented with hemoptysis and intermittent shortness of breath and was treated for NSCLC. Four months later, the patient presented again with an area of pneumonitis corresponding to fields of previous irradiation after being treated with durvalumab. Since RRP may mimic cancer progression or pneumonia, bronchoscopy ruled out both causes. Coupled with CT scan findings, it was therefore concluded that the pneumonitis was RRP induced by durvalumab. Systemic steroids and withdrawal of durvalumab promptly improved RRP with durable shrinkage in mass. The patient was started on high-dose steroids, and treatment with durvalumab was discontinued. Within days, the patient demonstrated symptomatic improvement and significant decrease in RUL consolidation.
Discussion. Non-small cell lung cancer is the most common type of lung cancer, accounting for about 85% of all cases.4 It is a complex and heterogeneous disease encompassing several subtypes, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Generally, NSCLC is diagnosed at an advanced stage, which poses a significant challenge for treatment and prognosis. One of the main risk factors for NSCLC is smoking, with about 85% of cases occurring in current or former smokers. Other risk factors include exposure to secondhand smoke, radon gas, asbestos, and air pollution. Genetic factors may also play a role in the development of NSCLC, as certain gene mutations have been associated with an increased risk of the disease.5 Common symptoms include persistent cough, chest pain, shortness of breath, and unintended weight loss. However, many patients with NSCLC may not experience any symptoms until the disease has reached an advanced stage, making early detection and diagnosis challenging.6 Treatment options for NSCLC depend on the stage of the disease and may include surgery, radiation therapy, chemotherapy, targeted therapy, immunotherapy, or a combination of these modalities. In recent years, significant advancements have been made in the treatment of NSCLC, particularly in the development of targeted therapies that specifically target the genetic mutations driving the growth of cancer cells. Despite these advancements, the prognosis for patients with NSCLC remains poor, with a 5-year survival rate of less than 20%. Early detection and diagnosis are crucial for improving outcomes in patients with NSCLC, underscoring the importance of continued research and innovation in the field of lung cancer. As our understanding of the molecular mechanisms underlying NSCLC continues to evolve, it is hoped that new, more effective treatment strategies will emerge, ultimately leading to improved outcomes for patients with this devastating disease.7
RRP is an uncommon but potentially serious adverse event that can occur in patients who have received radiation therapy and subsequently receive certain medications, including immunotherapy like Durvalumab. RRP typically manifests as inflammation in previously irradiated lung tissue triggered by the administration of certain drugs. It can lead to symptoms such as cough, fever, shortness of breath, and chest pain, which may mimic radiation pneumonitis but occur outside the radiation field.8 A history of exposure to such agents after thoracic radiotherapy, radiographic abnormality, and clinical presentation establishes the diagnosis of RRP.9
Durvalumab is a monoclonal antibody drug used to treat various types of cancer, including NSCLC. First approved by the FDA in 2018 for treating patients with unresectable Stage I NSCLC, it targets and inhibits a protein called programmed cell death ligand-1 (PD-L1) on cancer cells, which helps the immune system effectively recognize and destroy these abnormal cells. Durvalumab has shown promising results in clinical trials for various cancers, including lung and bladder cancer, and has been approved by the FDA for use in certain cases.10 Its mechanism of action and potential as an immunotherapy agent make it a valuable tool in the fight against cancer, offering new hope for patients with difficult-to-treat diseases. The development of durvalumab represents a significant advancement in the field of oncology, and ongoing research continues to explore its potential in combination with other therapies for an even greater impact on cancer treatment.11
Traditional chemotherapies are known to be associated with RRP, but the increasing use of immunotherapies such as durvalumab in the treatment of cancer is leading to the emergence of RRP. A literature review shows that RRP has been frequently described in patients treated with gemcitabine, nivolumab, everolimus, and sunitinib. However, RRP induced by durvalumab has been reported only a handful of times and is extremely rare.12 It's worth noting that while RRP is relatively rare, health care providers should be aware of this potential complication, especially when administering medications like durvalumab to patients who have received prior radiation therapy for various cancers.13
Conclusion. Early recognition and management can help mitigate the impact of RRP on patient outcomes. This case opens up a discussion about the potential need for further investigation of interactions between immune checkpoint inhibitors and radiotherapy.
AUTHORS:
Syed M Imam, DO1 • Faryal Haider, MD2 • Syed A. A. Rizvi, MD, PhD, MPH, MBA, FRSM3,4
AFFILIATIONS:
1HCA Northside Hospital, USF Morsani College of Medicine, St Petersburg, Florida, USA
2The Wright Center for Graduate Medical Education, Scranton, Pennsylvania, USA
3College of Biomedical Sciences, Larkin University, Miami, Florida, USA
4Division of Clinical & Translational Research, Larkin Community Hospital, Miami, Florida, USA
CITATION:
Imam SM, Haider F, Rizvi SAA. A rare case report on radiation recall pneumonitis in a patient treated with durvalumab for non-small cell lung cancer. Consultant. DOI: 10.25270/con.2024.12.000001
Received June 7, 2024. Accepted September 27, 2024.
DISCLOSURES:
The authors report no relevant financial relationships.
ACKNOWLEDGEMENTS:
None
CORRESPONDENCE:
Syed A. A. Rizvi., PhD, MD, MPH, MBA, FRSM. Larkin University, 18301 N Miami Ave, Miami, FL 33169 (srizvi@larkin.edu)
References
Nobashi TW, Nishimoto Y, Kawata Y, et al. Clinical and radiological features of immune checkpoint inhibitor-related pneumonitis in lung cancer and non-lung cancers. Br J Radiol. 2020;93(1115):20200409. doi:10.1259/bjr.20200409
Chen X, Sheikh K, Nakajima E, et al. Radiation versus immune checkpoint inhibitor associated pneumonitis: distinct radiologic morphologies. Oncologist. 2021;26(10):e1822-e1832. doi:10.1002/onco.13900
Gorospe L, Martín-Martín M, Paredes-Rodríguez P, Gómez-Rueda A. Radiation recall pneumonitis: PET/CT findings. Jpn J Clin Oncol. 2023;53(6):534-535. doi:10.1093/jjco/hyad013
Riely GJ, Wood DE, Ettinger DS, et al. Non-small cell lung cancer, version 4. 2024. J Natl Compr Canc Netw. 2024;22(4):249-274. doi:10.6004/jnccn.2204.0023
Okahisa M, Udagawa H, Matsumoto S, et al. Clinical outcomes in patients with non-small cell lung cancer harboring EGFR Exon20 in-frame insertions in the near-loop and far-loop: Results from LC-SCRUM-Asia. Lung Cancer. 2024;191:107798. doi:10.1016/j.lungcan.2024.107798
Clark SB, Alsubait S. Non–Small Cell Lung Cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing; September 4, 2023
Mithoowani H, Febbraro M. Non-small-cell lung cancer in 2022: a review for general practitioners in oncology. Curr Oncol. 2022;29(3):1828-1839. doi:10.3390/curroncol29030150
Jan PR, Chang JW, Wu CE. Radiation recall pneumonitis: a rare syndrome that should be recognized. Cancers (Basel). 2022;14(19):4642. doi:10.3390/cancers14194642
Grassi F, Granata V, Fusco R, et al. Radiation recall pneumonitis: the open challenge in differential diagnosis of pneumonia induced by oncological treatments. J Clin Med. 2023;12(4):1442. doi:10.3390/jcm12041442
Trinh JQ, Xiong Y, Smith LM, Abughanimeh O, Marr AS, Ganti AK. Durvalumab outcomes in stage iii non-small cell lung cancer: a single-institution study. Anticancer Res. 2024;44(2):605-612. doi:10.21873/anticanres.16849.
Tsuji K, Mizugaki H, Yokoo K, et al. Durvalumab after chemoradiotherapy in non-small cell lung cancer with EGFR mutation: A real-world study (HOT2101). Cancer Sci. 2024;115(4):1273-1282. doi:10.1111/cas.16094
Nakamura K, Okubo K, Takahashi T, Mitsumori K, Ishigaki T, Ohnishi H. Radiation recall pneumonitis induced by nivolumab in a patient with renal cell carcinoma. IJU Case Rep. 2018;2(1):30-33. doi:10.1002/iju5.12032
Jan PR, Chang JW, Wu CE. Radiation recall pneumonitis: a rare syndrome that should be recognized. Cancers. 2022;14(19):4642. doi:10.3390/cancers14194642


