Peer Reviewed
Delayed Complication of “Fish-Oil” Injection Into the Face Associated With COVID-19 Exposure
AFFILIATIONS:
1General Medical Officer, Naval Medical Center, San Diego, CA
2Department of Dermatology, Naval Medical Center, San Diego, CA
CITATION:
Delayed complication of fish-oil injection into the face associated with COVID-19 exposure. Consultant. 2023;63(5):e9. doi:10.25270/con.2023.04.000002
Received August 19, 2022. Accepted January 11, 2023. Published online April 13, 2023.
DISCLOSURES:
The authors report no relevant financial relationships.
ACKNOWLEDGEMENTS
Patient has provided informed consent to release the photos of her face.
CORRESPONDENCE:
Sama Alazami, DO, 34800 Bob Wilson Dr, San Diego, CA 92134 (alazawisama@gmail.com)
A 41-year-old woman with a history of fibromyalgia, migraine, and irritable bowel disease presented to the emergency department (ED) with a 1-day history of facial swelling and upper lip swelling associated with pain and pressure.
History. The patient received subcutaneous “fish-oil” injections into her forehead and bilateral cheeks in Mexico for the treatment of her fibromyalgia 6- to 7-weeks prior to presentation. She denied a history of drug or food allergy. Her medications included duloxetine 30 mg and pregabalin 150 mg, both taken orally daily. She denied using new medications recently.
She stated that the edema is most severe along the injection sites. The swelling started on the bilateral cheeks then rapidly spread to involve the upper lip and forehead (Figure 1). She denied swelling of the tongue or involvement of the airway.

Figure 1. Diffuse edema of face and upper lip
On review of symptoms, the patient denied trouble breathing or other respiratory symptoms, fever, chills, nausea, vomiting, and abdominal pain. On physical examination, diffuse moderate edema of her bilateral cheeks, nasolabial folds, upper and lower eyelids, forehead, and upper lip were noted. No edema of the tongue or uvula was present.
Differential Diagnoses. The differential diagnosis for our patient included granulomatous-type hypersensitivity reaction, which usually develops 21-28 days after exposure to a foreign substance and manifests as nodules.1 Bachour and colleagues described the case of a 34-year-old woman who developed severe edema of the lips and face 7 days after self-injecting the contents of a fish oil capsule. The biopsy showed lipogranulomatous inflammatory reaction consistent with foreign body material injection.2 Their patient was treated with systemic steroids and had progressive improvements.2
We do not think our patient had sclerosing lipogranuloma since the nodules resolved by the 2-month follow-up. We considered allergic contact dermatitis, but this diagnosis was less likely with absences of papules, plaques, vesicles, and lichenification. She also lacked pruritus, an essential symptom of contact dermatitis. Infectious etiology was unlikely given absence of erythema and the patient being afebrile in the acute and chronic course.
Treatment and Management. The patient’s symptoms and history were consistent with angioedema. She was given 40 mg oral prednisone and oral diphenhydramine 25 mg once in the ED. The patient was discharged with oral prednisone 40 mg for 7 days and was instructed to follow-up with a dermatology specialist.
Outcome and Follow-up. The edema improved after completing the prednisone taper (Figure 2), as noted at the 10-day follow-up at the dermatology clinic. Upon obtaining further history, she denied a dermatologic history of atopic dermatitis, rosacea, seborrheic dermatitis, and psoriasis. She reported being in contact with a COVID-19-positive family member 4 weeks prior to developing the angioedema. She received two doses of the Pfizer vaccine 7 months prior to developing the face edema. Physical examination revealed palpable skin-colored nodules on the median forehead, bilateral medial canthus (which was worst on the right), and nasolabial folds (Figure 3). The patient was recommended to avoid further facial injections and follow-up again in 4- to 6-weeks. At the 2-month follow-up, the patient had complete resolutions of nodules and edema. Therefore, a biopsy was not deemed necessary.
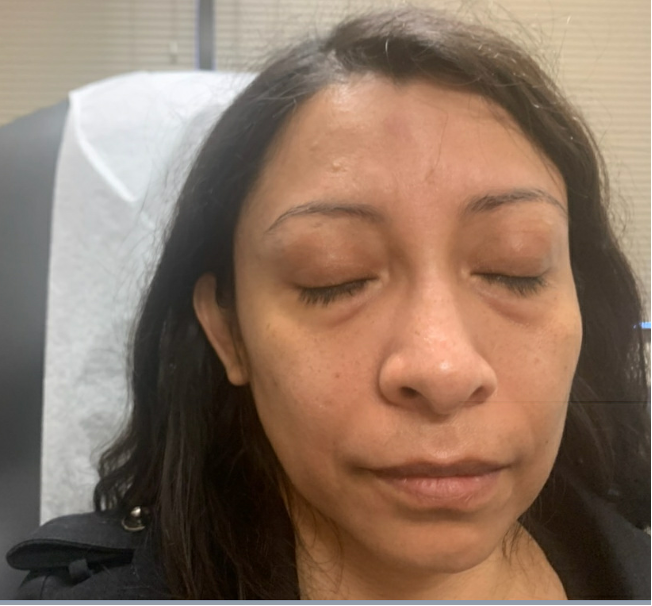
Figure 2. Resolution of edema at 10-day follow-up.
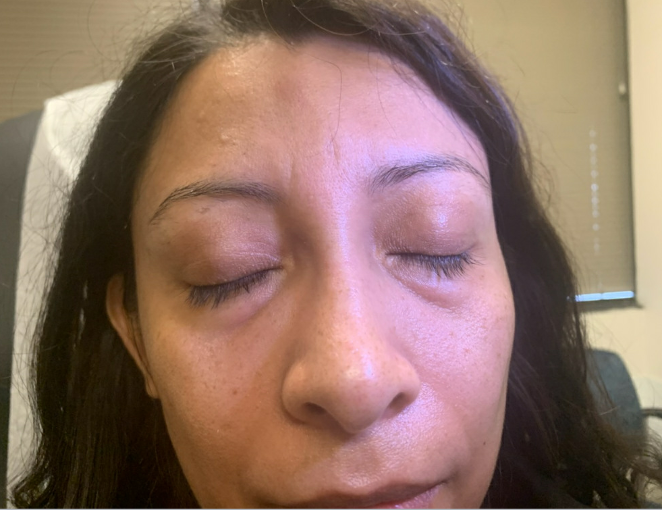
Figure 3. Palpable skin colored nodularity on the median forehead, bilateral medical canthus, and along the nasolabial folds at 10-day follow up.
Discussion. Oral fish oil consumption has many benefits including improving insulin sensitivity in patients with diabetes and reducing the risk of coronary heart disease by improving the lipid profile and blood pressure.3,4 However, subcutaneous fish-oil injection has not been authorized for medical use by the FDA.5 Few case reports have been published about subcutaneous injections of fish oil into different body parts including the face, lips, breast, and penis for off-label, cosmetic purposes. There are many complications associated with subcutaneous fish oil injections such as hypersensitivity reaction, infection, and granuloma formation. Injection of fish oil into the breast for the purpose of augmentation has led to inflammation and necrosis of the breast tissue.6 In another observational study, patients presented with complications from necrosis of penile skin to cellulitis and granuloma after subcutaneous injection of cod liver oil by non-medical personnel for penile augmentation.7 Additionally, subcutaneous fish-oil injection is not part of the medical therapy for fibromyalgia, as intended in our patient.8
Whether desperation or cost are driving some individuals into using off-label medicine, it is pertinent to encourage open-communications and discussions with patients regarding their plans and views of alternative treatments. This would aid in providing education to patients about the complications of different alternative treatments and influence them to make informed-medical decisions.
Delayed inflammatory reaction (DIR) is a term used to describe an inflammatory reaction to soft tissue filler and usually arises 2- to 4-weeks or longer post-injection.9 DIR is very similar to delayed hypersensitivity reaction but somewhat distinct in that a trigger or infectious etiology is needed to stimulate the inflammatory process.9 Delayed hypersensitivity reactions typically occur 12-72 hours after exposure and involve a T-cell mediated response to exogenous, hapten, or autoantigens.1,10 However, these reactions can be seen several weeks post-injection.10 DIRs following filler injections manifest as erythema, painful nodules, induration or tissue hardening, and solid edema.11
Several cases of inflammatory reactions have been associated with COVID-19 exposure in the literature. In the case series by Munavalli and colleagues, three out of four patients with a history of facial soft tissue fillers were at an increased risk of developing DIR, in the setting of COVID-19 vaccinations or infections.12 In one case, a 50-year-old woman developed severe swelling of the cheeks, lips, and tear troughs after testing positive for COVID-19 infection 15 days after her last hyaluronic acid (HA) filler injection.12 Additionally, a 36-year-old woman with a history of tear trough filler 2.5 years prior to presentation, had worsening bilateral infraorbital perioral edema 36 hours post-administration of the Moderna COVID-19 vaccine.12 Lastly, a 43-year-old woman developed periorbital edema 36 hours after administration of Pfizer vaccine.12 Usually, these patients responded well to oral and injectable steroids for treatment.12 The fourth case here was excluded because the patient, who had a history of HA fillers, developed facial edema 8 days after receiving a saline placebo in a Moderna COVID-19 vaccine clinical trial. Her COVID-19 antibody test was negative. Her reaction is likely due to the HA fillers.12 In another case report, a 55-year-old woman who had received dermal fillers 8 months prior developed angioedema of face and lips 13 days after COVID-19 infection.13
Although our patient had negative respiratory symptoms, she had a COVID-19 contact 4-weeks prior to developing the facial angioedema. Thus, she still could have had an asymptomatic COVID-19 infection.14 We believe that our patient developed a delayed angioedema and inflammatory reaction to the injected foreign body, possibly triggered by COVID-19 exposure, potential infection, and prior vaccination. Obtaining a prompt and thorough history regarding COVID-19 exposure or COVID-19 vaccination status can be an important tool towards accurate clinical diagnosis among clinicians.
Conclusion. Our patient developed delayed angioedema of the face secondary to fish-oil injections, likely triggered by a recent COVID-19 infection. Although fish oil injection is not authorized in the United States, patients can still receive off-label injections in a foreign country or through self-injections. Therefore, it is pertinent to understand the patients’ plans in approaching treatment, especially if their disorder is refractory to multiple therapies. Providing education about complications of off-label therapies would aid patients in making more informed-decisions.
- Justiz Vaillant AA, Zulfiqar H, Ramphul K. Delayed Hypersensitivity Reactions. StatPearls Publishing; 2022. Accessed July 29, 2022. https://www.ncbi.nlm.nih.gov/books/NBK519023/
- Bachour J, Sakkal M, Afif C, & Ammoury A. A case report of sclerosing lipogranulomatosis following fish oil Injection in lips. J Clin Exp Dermatol Res. 2019;10(2). doi:10.4172/2155-9554.1000492.
- Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27(7):885-896. Doi:10.1093/ajh/hpu024.
- Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9:345-381. doi:10.1146/annurev-food-111317-095850
- Krupa K, Fritz K, Parmar M. Omega-3 fatty acids. StatPearls Publishing; 2022. Accessed January 8, 2023. https://www.ncbi.nlm.nih.gov/books/NBK564314/
- Turk E, Karagulle E, Koksal H, et al. Bilateral breast necrosis due to local injection of fish oil. Breast J. 2013;19(2):196-198. doi:10.1111/tbj.12082
- Al-Ansari AA, Shamsodini A, Talib RA, Gul T, Shokeir AA. Subcutaneous cod liver oil injection for penile augmentation: review of literature and report of eight cases. Urology. 2010;75(5):1181-1184. doi:10.1016/j.urology.2009.11.023
- Chinn S, Caldwell W, Gritsenko K. Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep. 2016;20(4):25. doi:10.1007/s11916-016-0556-x
- Artzi O, Cohen JL, Dover JS, et al. Delayed inflammatory reactions to hyaluronic acid fillers: a literature review and proposed treatment algorithm. Clin Cosmet Investig Dermatol. 2020;13:371-378. doi:10.2147/CCID.S247171
- Bhojani-Lynch T. Late-onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open. 2017;5(12):e1532. doi:10.1097/GOX.0000000000001532
- Kim H, Cho SH, Lee JD, Kim HS. Delayed onset filler complication: two case reports and literature review. Dermatol Ther. 2017;30(5):e12513. doi:10.1111/dth.12513
- Munavalli GG, Guthridge R, Knutsen-Larson S, Brodsky A, Matthew E, Landau M. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2022;314(1):1-15. doi:10.1007/s00403-021-02190-6
- Virdi G. Dermal fillers and COVID-19: angioedema with urticaria in a patient post COVID-19 infection. Cureus. 2022;14(4):e24461. doi:10.7759/cureus.24461
- Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54(1):12-16. doi:10.1016/j.jmii.2020.05.001


