Visual Impairment in the Elderly: Impact on Functional Ability and Quality of Life
Visual impairment is one of the most feared forms of medical disability.There are two common definitions of visual impairment. In the United States, visual impairment is defined as visual acuity worse than 20/40 but better than 20/200 (legal blindness) in the better eye, even with corrective lenses.1,2 The World Health Organization defines visual impairment as visual acuity worse than 20/70 but better than 20/400 (legal blindness) in the better eye, even with corrective lenses.3
Visual impairment imposes a great social and economic burden on individuals and society alike. The total annual economic impact of visual impairment in adult Americans reaches approximately $51.4 billion, according to studies conducted independently by Rein et al4 and Frick et al.5 As the population ages and life expectancy increases, burgeoning healthcare costs will no doubt accompany this flourishing population. The US Census Bureau reported an average life expectancy of 78.3 years for 2010 and a projected average life expectancy of 79.5 years for 2020.6
Visual impairment disproportionately affects the elderly. A 1997 population-based study showed that the rate of visual impairment increased from 4% in persons age 65 years to 16% in those age ≥80 years, with an overall rate of 7%.7 The Eye Diseases Prevalence Research Group has reported age-related conditions such as age-related macular degeneration (AMD) and cataracts as the leading causes of visual impairment and blindness in the United States.8 The number of Americans at risk is expected to increase as longevity increases. Based on data from the 2000 US Census, Congdon et al8 found that 0.78% of the US population age >40 years was legally blind (≤20/200 in the better eye, even with corrective lenses) and an additional 1.98% had low vision (<20/40 with best correction). By 2020, the number of persons in the United States who are blind is expected to increase by 70%, with 1.6 million individuals affected, and the prevalence of persons with low vision is estimated to increase to 2.5%.8
Rates of blindness and visual impairment are expected to increase even further in disadvantaged, minority populations, particularly among African American and Latino subpopulations, which have an increased prevalence of diabetes and of hypertension. African Americans had an increased risk of developing primary open-angle glaucoma compared with whites in a large 1991 study conducted by the Wilmer Eye Institute.9 More recently, the anatomic microstructure of the posterior sclera in African Americans was found to be significantly different from that of whites, possibly favoring the earlier development and severity of ocular disease in African Americans.10 The LALES (Los Angeles Latino Eye Study),11 a major study conducted by the Doheny Eye Institute, found that the rates and severity of visual impairment, blindness, glaucoma, cataract, diabetic retinopathy, and macular degeneration were high in Latinos of predominantly Mexican descent living in Los Angeles, CA, when compared with non-Hispanic whites living in the United States.11-14 As minority populations expand and age, rates of visual impairment among these groups are also likely to grow.
Vision management in the expanding population of elderly persons in the United States presents a growing challenge to primary care physicians and subspecialists such as geriatricians and ophthalmologists. Despite the increasing number of older persons with visual impairment, delivery of adequate eye care to elderly individuals remains an unsolved problem. Surveys conducted in the United States have identified significant rates of untreated eye disease among elderly persons,15-17 with nursing home residents being notably underserved.18
The Centers for Disease Control and Prevention has declared age-related vision loss as a public health problem, citing the criteria posed by Saaddine et al19 as evidence.1 These criteria mirror those observed by the National Eye Institute20: vision loss affects a large elderly population; is a social and financial burden; is destined to increase in prevalence; is perceived as a threat to the public; and is preventable with action at the community level.
In this article, we review the anatomical and physiological changes that occur in the aging eye and include information on the leading causes of vision loss in the elderly. We also discuss medications with ocular side effects, such as amiodarone and busulfan, and examine the impact of visual impairment on elderly persons, including psychological and physical consequences, predisposition to motor vehicle accidents, quality of life, and mortality.
The Aging Eyes
As the eye ages, most of the anatomical and physiological processes gradually decline. The eyelids experience a loss of elasticity and tone, a common feature of senescent cutaneous tissues. The resulting involutional atrophy contributes to the etiology of several eyelid disorders, including entropion and ectropion. Tear production by the lacrimal gland may decrease with aging. This change, combined with an age-related decline in production of conjunctival mucin by the goblet cells and stabilizing surface oil by the Meibomian glands, may result in elderly individuals having dry eyes. The lens of the eye is also affected by advancing age. Age-related protein changes cause the lens to become denser, less elastic, larger, and yellow, leading to the development of cataracts. Many of these changes are thought to result from accumulative UV light effect on the structural proteins of the lens. The prevailing hypothesis emphasizes the role of oxidation and the formation of disulfide bonds in the pathogenesis of cataract. In addition to the above age-related changes, accommodation (the process by which the eye changes optical power to maintain a clear image [focus] on an object as its distance changes) decreases and vitreous gel tends to liquefy as one gets older. Finally, the retinal vasculature ages just like the vasculature in other parts of the body. Despite these eminent changes in the aging patient, however, many elderly individuals retain completely acceptable visual acuity.21
Visual acuity can be divided into static and dynamic types. Static acuity, the ability to resolve stationary details, declines with increasing age. Dynamic acuity, the ability to resolve the details of a moving target, also declines as one gets older. Detection of movement is also, to some extent, a function of luminance. The minimum amount of light needed for vision is known as the absolute threshold.
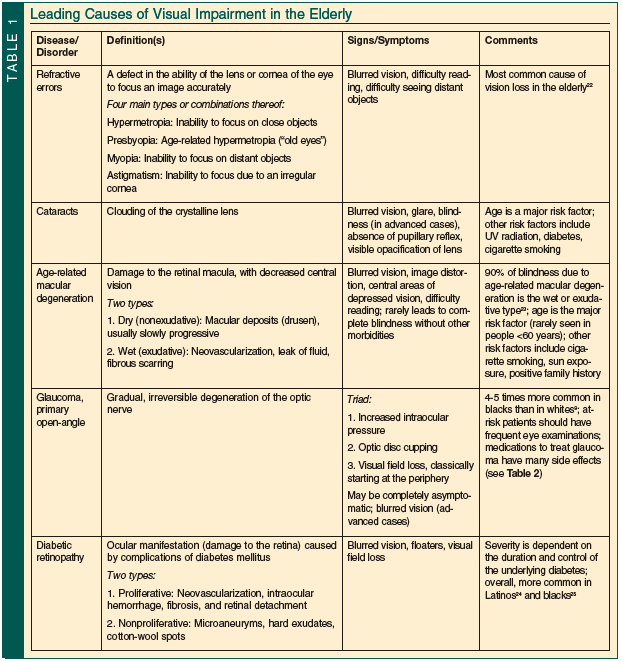
Table 1 summarizes the leading causes of visual impairment in the elderly.9,22-25
Medications and Their Correlation With Visual Impairment
Elderly patients frequently have altered drug distribution resulting from an increase in fat, loss of muscle, and changes in protein binding. They also often have altered drug metabolism, possibly from hepatic disease, and drug excretion, which can be caused by decreased renal function. Additionally, the number of drugs prescribed for elderly patients is often high, and noncompliance or misuse is a distressingly high occurrence in this population. Adverse drug reactions also occur more frequently in the older population (2%-10% in younger adults vs 20%-25% in elderly patients26). This section reviews some of the more common systemic medications with ocular side effects.
Amiodarone is used in the treatment of life-threatening arrhythmias. Ocular side effects of this drug were first described in the 1960s.27 The most common of these effects is cornea verticillata involving the deep epithelium. Other potential effects are lens opacities and anterior ischemic optic neuropathy. Busulfan, used in the treatment of chronic myeloid leukemia, may cause cataracts and corneal thinning. Chloroquine, used in the treatment of malaria, rheumatoid arthritis, and lupus erythematosus, has been linked with a “bull’s eye” maculopathy with impairment of central vision.28 Major antipsychotics, such as chlorpromazine, may cause mydriasis and interfere with accommodation.29 They may also cause a pigmentary retinopathy and granular deposits in the corneal endothelium or deep stroma with long-term use. Digitalis derivates such as digoxin may produce various visual disturbances, such as photopsias and decreased visual acuity, in addition to the classic “yellow discoloration” around objects.30 Ethambutol, used in the treatment of tuberculosis, can produce dyschromatopsia, optic atrophy, and visual field defects.31 Interferon alfa has been shown to cause retinopathies, such as cotton-wool spots and intraretinal hemorrhages, and has also been associated with optic disc edema and retinal vein occlusion.32 Ocular side effects of nitrofurantoin, used mainly in the treatment of urinary tract infections, include amblyopia, diplopia, nystagmus, and optic neuritis; retinopathy due to intraretinal crystals has also been reported.33 Sildenafil, prescribed to treat erectile dysfunction, cross-reacts with the retinal isoform of phosphodiesterase and may cause increased light sensitivity and transient perception of a bluish haze.34 The association between systemic steroid use and increased intraocular pressure has been well documented. These drugs are also notorious for accelerating the development of cataracts.35 Patients taking tamoxifen for breast cancer may develop retinopathy and visual impairment at high dosages.36 Finally, tricyclic antidepressants have a parasympatholytic action, which may cause mydriasis and paralysis of accommodation.37
In similitude to systemic medications affecting the eye, ophthalmic medications have been shown to affect systemic structures (Table 2).

Impact of Visual Impairment on the Elderly
The realization that one’s vision is deteriorating is often associated with psychological reactions such as grief, confusion, anger, fear, anxiety, diminished emotional security, and fluctuations in appetite.38 Older adults with new visual impairment(s) face a significant challenge at a time when they may also be experiencing other major life changes, such as general health limitations or the loss of a spouse. Loss of independence and the ability to enjoy leisure activities are predominant concerns for the older adult with a visual impairment.39 As his or her dependence upon others increases, it is not uncommon for the patient to experience loss of self-esteem and, in some cases, depression. A review of the clinical literature reveals that visual impairment is associated with a higher than normal risk of depression. Carabellese et al40 found that persons with vision impairments had a risk of depression 2.3 times greater than those without a vision problem. Horowitz et al41 also identified this association.
The physical consequences of visual impairment range across a very broad spectrum, from the seemingly innocuous hindrance in performing activities of daily living (ADLs) to more obvious, life-threatening effects such as accidents. ADLs and instrumental activities of daily living (IADLs) are greatly affected by visual impairment. Multiple studies conducted in different populations have demonstrated that a binocular visual acuity worse than 20/40 is an important predictor of functional loss in the elderly and is associated with social isolation (defined by patients as not attending social or religious activities).7,42,43 Binocular visual acuity worse than 20/40 has also been found to have a negative impact on IADLs, physical functioning, and social interaction.44 Patients with visual impairment were twice as likely as those without visual impairment to have difficulties with ADLs (eg, dressing, bathing, eating, getting in and out of bed) and IADLs (eg, housekeeping, grocery shopping, food preparation).42,43 Data also support the fact that the level of decline in performing such tasks is proportional to the level of decline in visual function.42,43 Nursing home staff should be aware of the relationship between decreased vision and an increased dependency for ADLs.45 Interventions to improve vision in older adult nursing home residents are successful in increasing visual function, performance of ADLs, and vision-targeted quality of life.
During the aging process, neuromuscular decline produces postural instability. This, coupled with the physiological changes in vision, predisposes elderly persons to falls. Studies have found that poor visual acuity,46 self-reported poor vision,47 visual field loss,48 poor stereoscopic vision,49 and impaired contrast sensitivity50 are significantly associated with falls. Longitudinal studies such as the Better Framingham Eye Study51 have demonstrated a relationship between poor vision and hip fracture, calculating the risk of hip fracture attributable to visual acuity at 18%. The Blue Mountains Eye Study52 confirmed that visual impairment is strongly associated with an increased risk of falls and hip fracture; poor visual acuity, the presence of a posterior subcapsular cataract, or the presence of a visual field defect were all associated with an increased risk of both falls and hip fracture. A prospective study of falls before and after cataract surgery showed that cataract removal is an effective intervention for reducing the risk of falls among elderly patients.53
The visually impaired elderly are not only at risk of accidental falls, but also of more serious accidents such as road traffic accidents. As individuals grow older, they suffer gradual and insidious changes in sensory efficiency. Because of the insidious nature of these changes, elderly drivers are at increased risk of being involved in a motor vehicle accident. A 1994 study by Holland and Rabbitt54 found that drivers in their 70s were not generally aware of the extent to which their visual acuity had declined, especially in poor light, and many drivers did not wear their prescribed spectacles appropriately.
McFarland et al55 reported in 1968 that older people require a greater absolute threshold than younger people in order to achieve equal improvement in the level of visibility of a target under different levels of illumination. The results of a more recent study by Sturr et al56 give insight into the vision problems that older drivers have in poor lighting. Researchers tested visual acuity at different illumination levels, going in small increments from highest illumination level to lowest illumination level and then from lowest to highest. Two minutes of adaptation time were allotted for everyone at the lowest luminance level. The results showed that 1.7% of persons age 18 to 25 years failed to see a 20/200 target as compared with 75% of persons age 60 to 87 years.56 This information has important implications for the issuance of driving permits to elderly individuals, indicating that nighttime visual acuity should not be assumed from the results of standard daytime testing.
Glare also becomes a problem for drivers as they age. This is mainly due to the increasing opacification of the lens with age. The effect of glare from the headlights of oncoming vehicles is of particular importance in the perception of road markings such as central white lines. Staplin et al57 found that on a wet road with markings having a retroreflective factor of 1.06, older drivers (age >65 years) could see white central street lines at a distance of 75 feet, whereas younger drivers (age 35 years) could see them at a distance of 110 feet. Researchers incorporated an oncoming vehicle with its headlights on into this experimental situation, and when the oncoming vehicle was 200 feet away, the older drivers could see the street lines at a distance of 30 feet, whereas younger drivers could see the street lines from a distance of 70 feet.57
The elderly have many other differences in their visual capabilities that have implications for safe driving, including decreased contrast sensitivity, restriction of visual fields, decreased visual localization, changes in detection and visual pursuit of a moving target, and age-related deficits in spatial ability. Contrast sensitivity measures seem to show a more reliable relationship with one’s ability to see signs and distinguish symbols, text, and faces, among others, than actual visual acuity.58 Older studies attempted to find an association between visual field narrowing and the risk of accidents in the elderly.59 Controversy loomed until more recent studies incorporated measures of peripheral attention with peripheral vision.60 This assessment became known as the useful field of view (UFOV). After assessing data from four cohort studies, Cross et al61 recently reported that falls and impaired UFOV were positively associated with overall risk of motor vehicle collisions.
It has long been demonstrated that older individuals tend to have difficulty identifying a target from among distracters. For example, Kosnik et al62 found that older people report difficulty in finding a friend in a crowd or in reading a street sign when other signs surround it. There are changes in the detection and visual pursuit of a target as an individual ages. Researchers found that the ability to detect movement (ie, movement lateral to the observer and in-and-out movement) declines in older persons.63,64 In road traffic terms, this means that older individuals are less able to detect movement and changes in movement of vehicles transversing across their visual field and also of vehicles straight ahead of them that are stopping, slowing, accelerating, and reversing.63,64
Given the significant vision changes that can occur in older persons and their implications on safe driving, it is not surprising that older drivers have a nine-fold increased risk of traffic fatality compared with younger adult drivers, as reported by the National Highway Traffic Safety Administration.65 In addition, the increased risks for falls and other accidents in elderly individuals with visual impairment make hospitalization more common in this group than in age-matched controls who are not similarly impaired. In a prospective study of hospital admissions among a population-based sample of community-dwelling adults age ≥75 years in Britain, Evans et al66 reported that older people who were visually impaired had 238.7 admissions/1000 person-years compared with 169.7 admissions/1000 person-years for older people with good vision. This can probably be attributed to higher rates of comorbidity and reduced functional ability in the visually impaired population.
Morse et al67 and Evans et al68 found an association between visual impairment and an extension of hospitalization by 2 days. This was after controlling for sex, age, and other comorbidities. Patients with visual impairment often have a bladder catheter inserted because of their difficulty in finding the bathroom and using the toilet appropriately.67 Catheterization predisposes patients to urinary tract infections, which, in turn, increases the length of hospital stay.67 Increased length of hospital stay in visually impaired patients can also be due, in part, to the development of delirium. It is well documented that visual impairment predisposes one to delirium,69 affecting 10% to 30% of hospitalized patients with medical illness and >50% of persons in certain high-risk populations.70 Considerable morbidity and mortality are associated with delirium. Patients with delirium have longer hospital stays and more medical complications, such as pneumonia and pressure ulcers. The mortality rate among elderly hospitalized patients with delirium is estimated to range from 22% to 76%.69 Sensory aids such as eyeglasses play an important role in the primary prevention of delirium and are associated with better outcomes.71
Quality of Life and Mortality
Numerous sources cite the significant impact of visual impairment on quality of life. Visual sensory impairment has been associated with depression, decreased efficiency in ADLs, and diminished social relationships.72,73 The Blue Mountains Eye Study74 reported that 24.5% of participants with normal vision, 35.5% of participants with mild visual impairment, and 48.8% of participants with moderate to severe visual impairment rated their health as poor or fair. Patients who have had cataract surgery have reported better health and quality of life and significant improvement in visual function status.75 Brenner et al76 reported that 1 year after cataract surgery, patients continued to experience improvement in the ability to carry out certain quality-of-life functions such as driving, and in community activities, mental health, and life satisfaction. Javitt et al77 found that the extraction of cataract from both eyes rather than from only one eye is the appropriate treatment for patients with bilateral cataract-induced visual impairment and provides the best opportunity for improvement in their quality of life.
Visual impairment has been associated with increased mortality, and many possible explanations for this increase have been suggested. Visual impairment inhibits elderly individuals’ ability to self-care and often has negative psychological effects. The visually impaired elderly patient is at a much greater risk of falls and other types of accidents such as burns. Visual impairment may influence medication compliance among elderly patients because of an inability to read standard print size on drug labels.78 In addition, ocular problems in the elderly are often markers of chronic medical conditions directly related to mortality risk. Multiple studies report an increased risk of death in elderly persons with visual impairment. The relationship between mortality and ocular disorders such as cataract, AMD, glaucoma, and diabetic retinopathy has been extensively investigated.79-86 It has been found that the presence of age-related cataract, either nuclear (relative risk [RR], 1.5), cortical (RR, 1.3), or posterior subcapsular cataract (RR, 1.5), is significantly associated with increased mortality risk.84 Some studies have shown that nuclear sclerosis is more highly associated with mortality risk than other types of cataract.85-88
The relation between AMD and mortality is not clear. The Copenhagen City Eye Study89 showed an increased mortality risk in women with age-related maculopathy but not in men. The Age-Related Eye Disease Study90 showed an increased risk of mortality in individuals with advanced-stage macular degeneration. The Blue Mountains Eye Study91 reported that AMD predicted mortality from stroke and cardiovascular events over the long term in persons age 49 to 75 years. Conversely, both the Beijing Eye Study92 and the Rotterdam Study84 reported that age-related maculopathy was not significantly associated with mortality.
Similar to AMD, the association between glaucoma and mortality remains controversial. A recent meta-analysis of nine cohort studies failed to demonstrate any significant association between primary open-angle glaucoma and all-cause or cardiovascular mortality.93 The Blue Mountains Eye Study,94 on the other hand, demonstrated an increased cardiovascular mortality in persons previously diagnosed with open-angle glaucoma. The Beijing Eye Study92 made a distinction between angle-closure glaucoma, which is associated with an increase in mortality, and open-angle glaucoma, which carries no such relationship.
In contrast to the unclear nature of the relationship between the two preceding conditions and mortality, studies have consistently demonstrated an association between diabetic retinopathy and increased mortality. This relationship is clearly described in the Wisconsin Epidemiologic Study of Diabetic Retinopathy.95 This is not surprising because retinopathy is also a marker for other microvascular changes affecting other organs. Studies examining the association between retinal vein occlusion (RVO) and mortality have found as much as a two-fold increase in the risk of cardiovascular mortality in persons with RVO.96,97
Discussion
As the population ages, the number of persons who are visually impaired is increasing, as is the need for appropriate evaluation, management, and rehabilitation for these individuals. Physicians, through their clinical education, training, and experience, are in a unique position to recognize the existence of, or the potential for, visual impairment in their patients. Despite the ophthalmologist’s role in the evaluation of these patients, it is critical that all sectors of primary care are aware of the factors associated with visual impairment in the elderly population. The American Academy of Ophthalmology recommends that older patients be evaluated at least once per year by an ophthalmologist.98 Despite this recommendation, 36% to 55% of the elderly population reported not having any eye services in more than a year.99-101 This alarmingly high percentage of nonattendance to eye care reflects a multifactorial problem, a significant part of which might be attributable to the low expectations regarding vision in elderly persons. Patients with systemic diseases such as diabetes or hypertension or with chronic eye diseases such as glaucoma or AMD should see their ophthalmologist even more frequently to prevent the emergence or worsening of visual impairment.
The use of eye care services by the older population has well-differentiated patterns along racial and gender lines. The SEE (Salisbury Eye Evaluation) project99 found that blacks were significantly less likely than whites to see any type of eye care provider over 1 year (50% vs 69%, respectively). This is of great concern because, as stated previously, African Americans are known to be at increased risk for several ocular conditions, such as primary open-angle glaucoma. In the Baltimore Eye Survey, Tielsch et al9 found that blacks had a prevalence of glaucoma four to five times that of whites, while Friedman et al102 found the age-adjusted prevalence of glaucoma to be nearly three times higher in blacks than in whites. Women are more likely than men to consult an ophthalmologist.101 Also, individuals with a higher level of education are more likely to present to an eye care provider.99 More studies are needed to determine the reasons behind the underuse of ophthalmic services by the elderly. The differences between genders and races must also be scrutinized and interventions put in place to remove barriers to access.
It is critical that the attending geriatrician be cognizant of the available options for vision rehabilitation. Vision rehabilitation is defined as “the process of treatment and education that helps individuals who are visually disabled attain maximum function, a sense of well-being, a personally satisfying level of independence, and optimum quality of life.”103 A literature review by the Agency for Healthcare Research and Quality concludes that “for vision rehabilitation, the research necessary for establishing evidence-based findings of effectiveness is only beginning to emerge.”104 To date, there have been no randomized controlled trials that have evaluated the effectiveness or cost-effectiveness of different models of care in patients with low vision. However, a study by McCabe et al105 that evaluated vision rehabilitation using optometry, occupational therapy, and social work showed significant improvement after vision rehabilitation in a predominantly elderly population.
Elderly patients who have regular eye examinations are less likely to experience a decline in vision status.106 Referral to the ophthalmologist is essential in cases of advanced clinical disease that require surgical intervention, such as cataracts. This diagnosis can usually be made from the bedside, and after surgery, follow-up is simple and efficient. Underserved patients, particularly minorities, often have limited treatment options because of the elevated cost of ophthalmic treatment. The nonprofit organization Vision Aware has compiled a comprehensive resource on local vision rehabilitation services, which is available on its Website (www.visionaware.org). This list is organized by state and includes local charity groups and social groups for blind individuals (www.visionaware.org/find_vision_rehabilitation_vision_services_in_your_state).
In many cases, treatment of an underlying condition can restore tolerable visual performance levels in the patient. Other situations require visual rehabilitation via use of low-vision aids. However, the evidence for the effectiveness of vision rehabilitation, though promising, needs to be expanded. Awareness of visual impairment is important to each of us as individuals and to society as a whole. Our communities must be educated on the importance of timely screening and annual follow-up. We must be cognizant of our own personal risk and take steps to preserve and protect the eyesight of our elders.
Acknowledgment
Supported in part by a departmental Challenge Grant from Research to Prevent Blindness Inc, New York, NY.
The authors report no relevant financial relationships.
References
1. Centers for Disease Control and Prevention. Why is vision loss a public health problem? http://www.cdc.gov/visionhealth/basic_information/vision_loss.htm. Accessed June 29, 2011.
2. American Foundation for the Blind. Living with low vision. http://www.afb.org/Section.asp?SectionID=26&TopicID=144. Accessed June 29, 2011.
3. World Health Organization. Prevention of blindness and visual impairment. ICD update and revision platform: change the definition of blindness. http://www.who.int/blindness/en/. Accessed June 29, 2011.
4. Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754-1760.
5. Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125(4):544-550.
6. U.S. Census Bureau. Statistical abstract of the United States: 2011. http://www.census.gov/compendia/statab/2011/tables/11s0103.pdf. Accessed June 29, 2011.
7. West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults. The SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38(1):72-82.
8. Congdon N, O’Colmain B, Klaven CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485.
9. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266(3):369-374.
10. Yan D, McPheeters S, Johnson G, Utzinger U, Vande Geest JP. Microstructural differences in the human posterior sclera as a function of age and race. Invest Ophthalmol Vis Sci. 2011;52(2):821-829.
11. Varma R, Paz SH, Azen SP, et al; Los Angeles Latino Eye Study Group. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111(6):1121-1131.
12. Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP; Los Angeles Latino Eye Study Group. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(7):1288-1297.
13. Varma R, Ying-Lai M, Klein R, Azen SP; Los Angeles Latino Eye Study Group. Prevalence and risk indicators of visual impairment and blindness in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(6):1132-1140.
14. Varma R, Choudhury F, Klein R, Chung J, Torres M, Azen SP; Los Angeles Latino Eye Study Group. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752-761.e1-3.
15. Tielsch JM, Sommer A, Witt K, Katz J, Royall RM. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108(2):286-290.
16. Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310-1315.
17. Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980;24(Suppl):335-610.
18. Tielsch JM, Javitt JC, Coleman A, Katz J, Sommer A. The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N Engl J Med. 1995;332(18):1205-1209.
19. Saaddine JB, Narayan KM, Vinicor F. Vision loss: a public health problem? Ophthalmology. 2003;110(2):253-254.
20. Prevent Blindness America, National Eye Institute. Vision problems in the U.S.—prevalence of adult vision impairment and age-related eye disease in America. 2008 update to the fourth edition. http://www.preventblindness.org/vpus/2008_update/VPUS_2008_update.pdf. Accessed July 6, 2011.
21. Teasdale TA. Self-Instructional Modules in Geriatric Medicine, 4th ed [CD]. Houston, TX: Baylor College of Medicine; 2000.
22. Centers for Disease Control and Prevention. Vision Health Initiative (VHI). National data. http://www.cdc.gov/visionhealth/data/national.htm. Accessed June 29, 2011.
23. Ferris FL III, Fine SL, Hyman LA. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640-1642.
24. Varma R, Torres M, Peña F, Klein R, Azen SP; Los Angeles Latino Eye Study Group. The prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(7):1298-1306.
25. Leske MC, Wu SY, Hyman L, et al. Diabetic retinopathy in a black population: the Barbados Eye Study. Ophthalmology. 1999;106(10):1893-1899.
26. Chutka DS, Evans JM, Fleming KC, et al. Symposium on geriatrics—part I: drug prescribing for elderly patients. Mayo Clin Proc. 1995;70(7):685-693.
27. Mantyjarvi M, Tuppurainen K, Ikaheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998;42(4):360-366.
28. Elman A, Gullberg R, Nilsson E, Rendahl I, Wachtmeister L. Chloroquine retinopathy in patients with rheumatoid arthritis. Scand J Rheumatol. 1976;5(3):161-166.
29. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501-526. doi:10.2165/11533180-000000000-00000.
30. Robertson DM, Hollenhorst RW, Callahan JA. Ocular manifestations of digitalis toxicity. Discussion and report of three cases of central scotomas. Arch Ophthalmol. 1966;76(5):640-645.
31. Carr RE, Henkind P. Ocular manifestations of ethambutol, toxic amblyopia after administration of an experimental antituberculous drug. Arch Ophthalmol. 1962;67:566-571.
32. Kawano T, Shigehira M, Uto H, et al. Retinal complications during interferon therapy for chronic hepatitis C. Am J Gastroenterol. 1996;91(2):309-313.
33. Ibanez HE, Williams DF, Boniuk I. Crystalline retinopathy associated with long-term nitrofurantoin therapy. Arch Ophthalmol. 1994;112(3):304-305.
34. Pepip S, Pitha-Rowe I. Stepwise decline in visual field after serial sildenafil use. J Neuroophthalmol. 2008;28(1):76-77.
35. Crews SJ. Posterior subcapsular lens opacities in patients on longterm corticosteroid therapy. Br Med J. 1963;1(5346):1644-1647.
36. Kaiser-Kupfer MI, Lippman ME. Tamoxifen retinopathy. Cancer Treat Rep. 1978;62(3):315-320.
37. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501-526. doi:10.2165/11533180-000000000-00000.
38. Branch LG, Horowitz A, Carr C. The implications for everyday life of incident self-reported visual decline among people over age 65 living in the community. Gerontologist. 1989;29(3):359-365.
39. Ringering L, Amaral P. The role of psychosocial factors in adaptation to visual impairment and rehabilitation outcomes for adults and older adults. In: Silverstone B, Lang MA, Rosenthal BP, et al, eds. The Lighthouse Handbook on Vision Impairment and Vision Rehabilitation. New York, NY: Oxford University Press, Inc.; 2000:1029-1048.
40. Carabellese C, Appollonio I, Rozzini R, et al. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc. 1993;41(4):401-407.
41. Horowitz A, Reinhardt JP, Boerner K. The effect of rehabilitation on depression among visually disabled older adults. Aging Ment Health. 2005;9(6):563-570.
42. Lee P, Smith JP, Kington R. The relationship of self-rated vision and hearing to functional status and well-being among seniors 70 years and older. Am J Ophthalmol. 1999;127(4):447-452.
43. Reuben DB, Mui S, Damesyn M, Moore AA, Greendale GA. The prognostic value of sensory impairment in older persons. J Am Geriatr Soc. 1999;47(8):930-935.
44. Sloan FA, Ostermann J, Brown DS, Lee PP. Effects of changes in self-reported vision on cognitive, affective, and functional status and living arrangements among the elderly. Am J Ophthalmol. 2005;140(4):618-627.
45. Marx MS, Werner P, Cohen-Mansfield J, Feldman R. The relationship between low vision and performance of activities of daily living in nursing home residents. J Am Geriatr Soc. 1992;40(10):1018-1020.
46. Clark RD, Lord SR, Webster IW. Clinical parameters associated with falls in an elderly population. Gerontology. 1993;39(2):117-123.
47. Lord SR, Ward JA, Williams P, Anstey KJ. An epidemiological study of falls in older community-dwelling women: the Randwick falls and fractures study. Aust J Public Health. 1993;17(3):240-245.
48. Glynn RJ, Seddon JM, Krug JH Jr, Sahagian CR, Chiavelli ME, Campion EW. Falls in elderly patients with glaucoma. Arch Ophthalmol. 1991;109(2):205-210.
49. Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261(18):2663-2668.
50. Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc. 1991;39(12):1194-1200.
51. Felson DT, Anderson JJ, Hannan MT, Milton RC, Wilson PW, Kiel DP. Impaired vision and hip fracture. The Framingham Study. J Am Geriatr Soc. 1989;37(6):495-500.
52. Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998;46(1):58-64.
53. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
54. Holland CA, Rabbitt PM. The problems of being an older driver: comparing the perceptions of an expert group and older drivers. Appl Ergon. 1994;25(1):17-27.
55. McFarland RA, Ryan GA, Dingman R. Etiology of motor-vehicle accidents, with special reference to the mechanisms of injury. N Engl J Med. 1968;278(25):1383-1388.
56. Sturr JF, Kline GE, Taub HA. Performance of young and older drivers on a static acuity test under photopic and mesopic luminance conditions. Hum Factors. 1990;32(1):1-8.
57. Staplin L, Breton ME, Haimo SF, Farber EI, Byrnes AM. Age related diminished capacities and driver performances. 1988. Contract No. DTFH 61-86-c-00044 FHA.
58. Owsley C, Sloane, ME. Contrast sensitivity, acuity and the perception of ‘real world’ targets. Br J Opthalmol. 1987;71(10):791-796.
59. Johnson CA, Keltner JL. Incidence of visual field loss in 20,000 eyes and its relationship to driving performance. Arch Ophthalmol. 1983;101(3):371-375.
60. Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34(11):3110-3123.
61. Cross JM, McGwin G Jr, Rubin GS, et al. Visual and medical risk factors for motor vehicle collision involvement among older drivers. Br J Ophthalmol. 2009;93(3):400-404.
62. Kosnik W, Winslow L, Kline D, Rasinski K, Sekuler R. Visual changes in daily life throughout adulthood. J Gerontol. 1988;43(3):P63-P70.
63. Shinar D, Schieber F. Visual requirements for safety and mobility of older drivers. Hum Factors. 1991;33(5):507-519.
64. Shinar D, Dewar R, Summala H, Zakowska L. Traffic sign symbol comprehension: a cross-cultural study. Ergonomics. 2003;46(15):1549-1565.
65. National Highway Traffic Safety Administration. Traffic safety facts 2008 data. Older population. http://www-nrd.nhtsa.dot.gov/Pubs/811161.PDF. Accessed June 27, 2011.
66. Evans JR, Smeeth L, Fletcher AE. Hospital admissions in older people with visual impairment in Britain. BMC Ophthalmol. 2008;8:16.
67. Morse AR, Yatzkan E, Berberich B, Arons RR. Acute care hospital utilization by patients with visual impairment. Arch Ophthalmol. 1999;117(7):943-949.
68. Evans JR, Fletcher AE, Wormald RPL, et al. Prevalence of visual impairment in people aged 75 years and older in Britain: results from the MRC trial of assessment and management of older people in the community. Br J Ophthalmol. 2002;86(7):795-800.
69. Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474-481.
70. Gleason OC. Delirium. Am Fam Physician. 2003;67(5):1027-1034.
71. Nakasato Y, Servat JJ, Amador F, Teasdale TA. Delirium in the older hospitalized patient. J Okla State Med Assoc. 2005;98(3):113-116.
72. Carabellese C, Appollonio I, Rozzini R, et al. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc. 1993;41(4):401-407.
73. Appollonio I, Carabellese C, Frattolla L, Trabucchi M. Effects on sensory aids on the quality of life and mortality of elderly people: a multivariate analysis. Age Ageing. 1996;25(2):89-96.
74. Wang JJ, Mitchell P, Smith W. Vision and low self-rated health: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2000;41(1):49-54.
75. Mangione CM, Phillips RS, Lawrence MG, Seddon JM, Orav EJ, Goldman L. Improved visual function and attenuation of declines in health-related quality of life after cataract extraction. Arch Ophthalmol. 1994;112(11):1419-1425.
76. Brenner MH, Curbow B, Javitt JC, Legro MW, Sommer A. Vision change and quality of life in the elderly. Response to cataract surgery and treatment of other chronic ocular conditions. Arch Ophthalmol. 1993;111(5):680-685.
77. Javitt JC, Brenner MH, Curbow B, Legro MW, Street DA. Outcomes of cataract surgery. Improvement in visual acuity and subjective visual function after surgery in the first, second, and both eyes. Arch Ophthalmol. 1993;111(5):686-691.
78. Watanabe RK, Gilbreath K, Sakamoto CC. The ability of the geriatric population to read labels on over-the-counter medication containers. J Am Optom Assoc. 1994;65(1):32-37.
79. Clemons TE, Kurinij N, Sperduto RD; AREDS Research Group. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13. Arch Ophthalmol. 2004;122(5):716-726.
80. Klein R, Klein BE, Moss SE. Age-related eye disease and survival. The Beaver Dam Eye Study. Arch Ophthalmol. 1995;113(3):333-339.
81. Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophthalmol. 2002;120(11):1544-1550.
82. McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol. 2001;85(3):322-326.
83. Wang JJ, Mitchell P, Simpson JM, Cumming RG, Smith W. Visual impairment, age related cataract, and mortality. Arch Ophthalmol. 2001;119(8):1186-1190.
84. Borger PH, van Leeuwen R, Hulsman CA, et al. Is there a direct association between age-related eye diseases and mortality? The Rotterdam Study. Ophthalmology. 2003;110(7):1292-1296.
85. Hennis A, Wu SY, Li X, Nemesure B, Leske MC; Barbados Eye Study Group. Lens opacities and mortality: the Barbados Eye Studies. Ophthalmology. 2001;108(3):498-504.
86. Thompson JR, Sparrow JM, Gibson JM, Rosenthal AR. Cataract and survival in an elderly nondiabetic population. Arch Ophthalmol. 1993;111(5):675-679.
87. West SK, Munoz B, Istre J, et al. Mixed lens opacities and subsequent mortality. Arch Ophthalmol. 2000;118(3):393-397.
88. Williams SL, Ferrigno L, Mora P, Rosmini F, Maraini G. Baseline cataract type and 10-year mortality in the Italian-American Case-Control Study of age-related cataract. Am J Epidemiol. 2002;156(2):127-131.
89. Buch H, Vinding T, la Cour M, Jensen GB, Prause JU, Nielsen NV. Age-related maculopathy: a risk indicator for poorer survival in women: the Copenhagen City Eye Study. Ophthalmology. 2005;112(2):305-312.
90. Clemons TE, Kurinij N, Sperduto RD; AREDS Research Group. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13. Arch Ophthalmol. 2004;122(5):716-726.
91. Tan JS, Mitchell P, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2007;114(6):1143-1150.
92. Xu L, Wang YX, Wang J, Jonas JJ. Mortality and ocular diseases: the Beijing Eye Study. Ophthalmology. 2009;116(4):732-738.
93. Akbari M, Akbari S, Pasquale, LR. The association of primary open-angle glaucoma with mortality: a meta-analysis of observational studies. Arch Ophthalmol. 2009;127(2):204-210.
94. Lee AJ, Wang JJ, Kifley A, Mitchell P. Open-angle glaucoma and cardiovascular mortality: the Blue Mountains Eye Study. Ophthalmology. 2006;113(7):1069-1076.
95. Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81(9):1158-1162.
96. Cugati S, Wang JJ, Knudtson MD, et al. Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology. 2007;114(3):520-524.
97. Xu L, Liu WW, Wang YX, Yang H, Jonas JB. Retinal vein occlusions and mortality: the Beijing Eye Study. Am J Ophthalmol. 2007;144(6):972-973.
98. American Academy of Ophthalmology. Comprehensive adult medical eye evaluation—October 2010. http://one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=64e9df91-dd10-4317-8142-6a87eee7f517#section1. Accessed June 29, 2011.
99. Orr P, Barrón Y, Schein OD, Rubin GS, West SK. Eye care utilization by older Americans: the SEE Project. Salisbury Eye Evaluation. Ophthalmology. 1999;106(5):904-909.
100. Ellwein LB, Friedlin V, McBean AM, Lee PP. Use of eye care services among the 1991 Medicare population. Ophthalmology. 1996;103(11):1732-1743.
101. Chiang YP, Wang F, Javitt JC. Office visits to ophthalmologists and other physicians for eye care among the U.S. population, 1990. Public Health Rep. 1995;110(2):147-153.
102. Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532-538.
103. American Optometric Association. Definition of vision rehabilitation. http://www.aoa.org/x6207.xml. Accessed June 27, 2011.
104. Agency for Healthcare Research and Quality. Behavioral modification and social support. http://www.ahrq.gov/clinic/vision/vision3.htm. Accessed June 27, 2011.
105. McCabe P, Nason F, Demers Turco P, Friedman D, Seddon JM. Evaluating the effectiveness of a vision rehabilitation intervention using an objective and subjective measure of functional performance. Ophthalmic Epidemiol. 2000;7(4):259-270.
106. Sloan FA, Picone G, Brown DS, Lee PP. Longitudinal analysis of the relationship between regular eye examinations and changes in visual and functional status. J Am Geriatr Soc. 2005;53(11):1867-1874.


