Treatment Options in Chronic Obstructive Pulmonary Disease: Current Recommendations and Recent Findings
ABSTRACT: Pharmacologic treatments and pulmonary rehabilitation improve symptoms, health status, and exercise tolerance in patients with chronic obstructive pulmonary disease (COPD). Short-acting and long-acting bronchodilators are the mainstays of therapy for as-needed symptom relief and long-term maintenance, respectively. Disease severity, device characteristics, medication costs, and patient preferences are factors to consider when making pharmacologic treatment decisions. Changes in symptoms, adherence to treatment, and existence of comorbidities should be assessed when evaluating the effectiveness of pharmacologic therapy. Pulmonary rehabilitation, including exercise training, nutritional counseling, psychosocial support, and patient education, has shown clinical benefits at all COPD severity stages. Oxygen therapy and surgical options may be considered in carefully selected patients. Research is ongoing to develop novel therapies that can modify disease progression and to identify COPD phenotypes for greater individualization of therapy. This article reviews current COPD treatment recommendations and potential novel COPD treatment options for the future.
_______________________________________________________________________________________________________________________________________
Patients with chronic obstructive pulmonary disease (COPD) can exhibit a significant response to bronchodilator therapy1-5; as such, short-acting and long-acting bronchodilator therapies are the mainstays of COPD treatment.6 Goals of pharmacotherapy and other elements of COPD management (eg, pulmonary rehabilitation, smoking cessation) are to relieve symptoms; prevent disease progression; improve exercise tolerance, health status, and quality of life; prevent and treat complications and exacerbations; and reduce mortality.6 Identification of comorbidities, which can complicate treatment and result in poor outcomes, also is necessary.6 COPD treatment is selected based on severity and
exacerbation frequency, clinical presentation, speed of lung function
decline, extrapulmonary effects, comorbidities, and patient factors.7
PHARMACOLOGIC TREATMENT OPTIONS
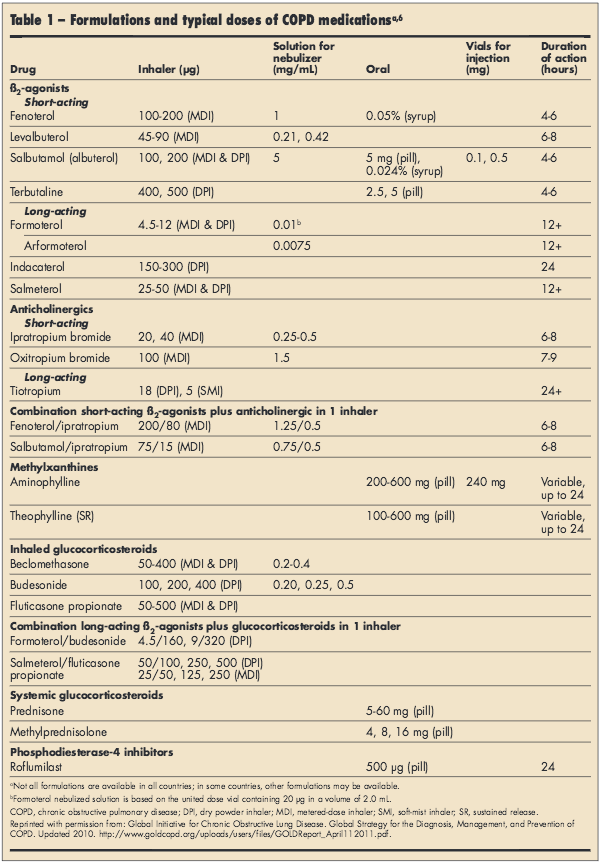
Table 1 summarizes available COPD medications.6 Regardless of other therapies, all COPD patients should have short-acting ß2-adrenergic agonists (SABAs) and short-
acting anticholinergics available for rapid symptom relief.6 SABAs relax airway smooth muscles,6 while short-acting anticholinergics block the bronchoconstrictive effects of acetylcholine on airway smooth muscle cells.6,8
Long-acting bronchodilators, including long-acting muscarinic antagonists (LAMAs) and long-acting ß2-adrenergic agonists (LABAs), are the mainstays of therapy for long-term COPD symptom management.6 Despite concerns stemming from asthma studies,9-11 the safety of LAMAs and LABAs has been shown in patients with COPD.8,12-14 The Understanding Potential Long-term Impacts on Function With Tiotropium (UPLIFT) study and a subsequent meta-analysis of 19 studies with tiotropium, including UPLIFT, showed no evidence of an increased risk of cardiovascular events.12,13 The safety of LABA monotherapy in COPD is well documented8,14; LAMAs and LABAs can be administered alone or as combination therapy for bronchodilation and reduction of symptoms and exacerbations.6,14
Regular inhaled corticosteroid (ICS) therapy may further reduce exacerbation frequency for patients with severe or very severe COPD and a history of frequent exacerbations.6 ICS treatment has not been proven to slow lung function decline in patients with COPD,6,15-18 but Towards a Revolution in COPD Health (TORCH) post hoc data suggest a slowing of lung function decline that should be further evaluated.19 ICS use in persons with COPD has been shown to increase the risk of pneumonia, which may vary by ICS type,6,20-23 and high-dose ICS therapy increases fracture risk.20 No increased risk of pneumonia was seen with inhaled budesonide (up to 640 µg/d) compared with placebo or formoterol alone over 12 months.24 Oral corticosteroids are not recommended for long-term therapy in patients with COPD.6 However, a 7- to 10-day burst of oral prednisone is a mainstay of COPD exacerbation therapy.6
HOW TO SELECT PHARMACOLOGIC THERAPY
Treatments should be selected based on the patient’s disease severity and COPD exacerbation frequency (Figure 1).6 The type of delivery device25 (eg, pressurized metered-dose inhaler, dry powder inhaler, or nebulizer), the skills and ability of the individual patient,6 the cost of each treatment (commonly US$36 to $210 per month26), and the patient’s financial status also should be considered. Patients with COPD routinely take 4 to 10 medications daily for various indications.27,28 Use of multiple types of inhaler devices can lead to confusion and errors in medication use29,30; therefore, it may be advantageous for physicians to keep the device consistent if possible.30 When used correctly, the device does not affect treatment efficacy31; however, it is important to ensure proper inhaler technique. At each clinic visit, a clinical team member should recheck the patient’s inhaler technique to ensure correct inhaler use.6 Information about inhaler devices, inhalation technique, and recommendations for training can be found here: http://advanceweb.com/web/AstraZeneca/focus_on_copd_issue3_DevicesForAerosol/focus_on_copd_issue3.html.32
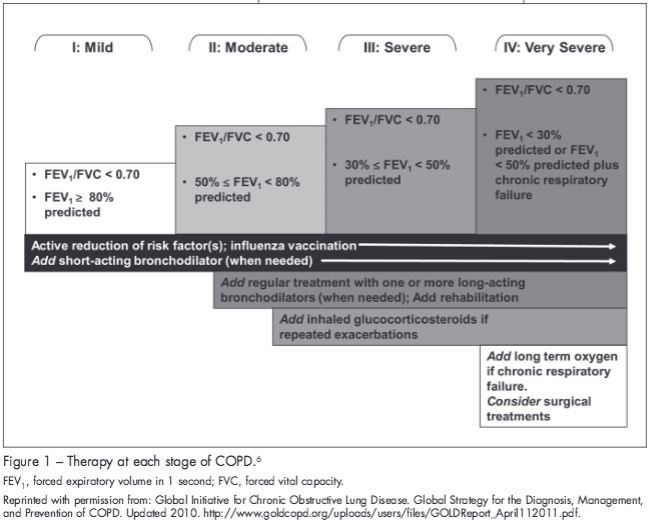
CHANGING PHARMACOLOGIC THERAPY
Patients with COPD should be monitored carefully for treatment response and side effects,6 and therapy should be individualized.6 Over time, all patients will require additional treatments or an increase in dose as disease severity worsens.6 However, COPD progression may not be recognized by patients over time.33 It is important for physicians to evaluate patients for signs and symptoms of disease worsening,33 including decreased exercise tolerance (what can the patient not do now that he/she could do last year?), impaired quality of life (what does the patient want to be able to do?), increased breathlessness at rest or during exercise (how many stairs can the patient go up before having to stop?), increased sputum (tablespoon versus a shot glass versus a cup full in a day), low or reduced oxygen saturation (pulse oximetry testing), change in ability to perform activities of daily living independently, and increased wheeze.34
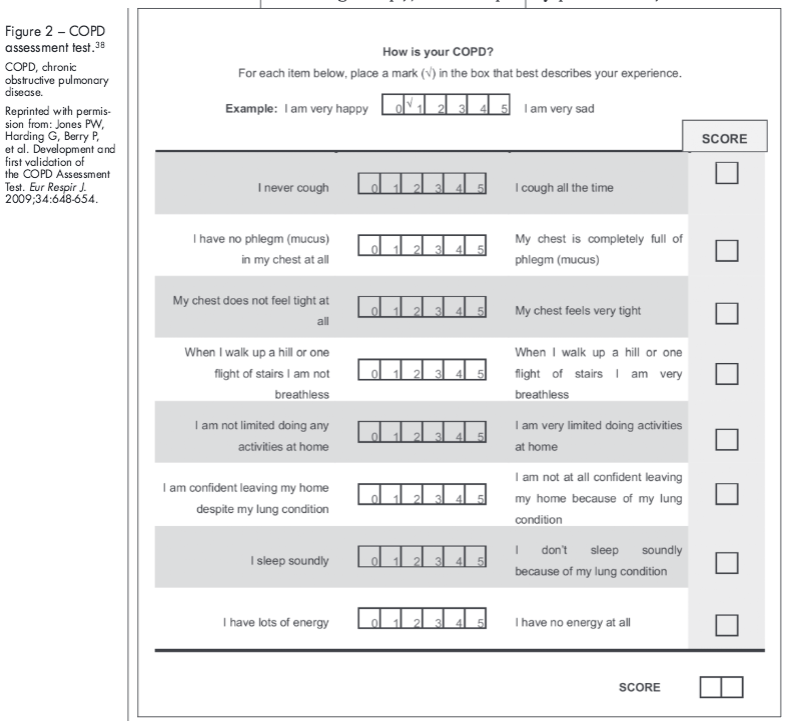
Assessment tools may help disease evaluations by providing physicians with clear and validated questions to ask patients. The Modified Medical Research Council (MMRC) dyspnea scale35 (http://copd.about.com/od/copdbasics/a/MMRCdyspneascale.htm) and the modified Borg scale36,37 (http://www.yourlunghealth.org/testing/6min_walk/index.cfm) can be used to follow disease progression if used repeatedly over time. The 8-item COPD Assessment Test (CAT) can be used to measure overall COPD-related health status (Figure 2),38 and the 14-item Exacerbations of Chronic Pulmonary Disease Tool-Patient-Reported Outcome (EXACT-PRO)39,40 (http://www.exactproinitiative.com/default.php#) can be used to quantify the severity of a COPD exacerbation.39
Continued on next page
IMPORTANCE OF PATIENT ADHERENCE TO COPD MANAGEMENT
COPD requires life-long medication therapy, and adherence to therapy is critical to achieving treatment goals. Poor adherence is common in patients with COPD, and adherence tends to decline over time.41,42 Thus, efforts to improve treatment adherence are worthwhile. Data from the TORCH study showed that good adherence (> 80% of doses) to inhaled COPD medication is associated with a significantly decreased risk of mortality and hospital admission for severe exacerbations over 3 years.43
Factors related to the patient (eg, health beliefs, self-efficacy, comorbidities, psychologic profile), the treatment (eg, dosing regimen, polypharmacy, side effects, method of administration), society (eg, social support, patient-prescriber relationship, access to medication, inhaler technique training), and health professionals (eg, education, goal setting, regular follow-up visits) can affect adherence.41 In particular, depression is a major contributor to poor adherence in patients with COPD, and its treatment substantially improves quality of life.44 Questionnaires including the Hospital Anxiety and Depression Questionnaire44 and the Patient Health Questionnaire-945 can help to identify patients who have significant anxiety and depression.
PULMONARY REHABILITATION
Pulmonary rehabilitation improves exercise capacity and health-related quality of life and reduces hospitalizations, perceived dyspnea intensity, anxiety, and depression in patients across all COPD severity levels.6 Components include exercise training, nutrition counseling, psychosocial support, and education.6,46 Exercise training programs generally range from 4 to 10 weeks, with greater benefits observed with longer programs.6,44 Strength training is especially important for patients with substantial muscle atrophy,44 while inspiratory muscle training may improve inspiratory muscle strength and decrease dyspnea.47 As part of nutrition counseling, physicians should ensure that patients with COPD who are underweight have adequate caloric intake and overweight patients have a diet that encourages fat loss.46 Maintenance of pulmonary rehabilitation is important, as benefits wane without continued exercise.6
LONG-TERM OXYGEN THERAPY
Patients with very severe COPD may require oxygen therapy to ensure that vital organs receive adequate oxygen.6 Supplemental oxygen therapy improves exercise capacity, ventilation, and neuropsychological performance (ie, memory and learning).48 The need for long-term oxygen therapy (> 15 hours per day), which increases survival in patients with chronic respiratory failure,6 is determined by the patient’s arterial blood gas values. Treatment goals include increased baseline partial pressure of arterial oxygen to ≥ 60 mm Hg and/or production of ≥ 90% oxygen saturation.6
SURGICAL TREATMENTS AND NEW AND EMERGING PHARMACOLOGIC TREATMENTS
Certain surgical treatments may be considered in carefully selected patients. Bullectomy, which involves removal of a large bulla in select patients with bullous emphysema, decompresses pulmonary parenchyma and improves pulmonary function and dyspnea.6 Lung volume reduction surgery improves expiratory flow rate by reducing hyperinflation and increasing lung elastic recoil.6 Compared with optimal medical therapy for severe COPD, lung volume reduction surgery has been shown to reduce the frequency of COPD exacerbations and increase the time to first exacerbation49; it also has been shown to decrease mortality in patients with upper-lobe predominant COPD and low exercise capacity.50 However, this costly procedure is recommended only in carefully selected patients.6 Lung transplantation is a rare therapy for COPD.6
Combinations of existing therapies and new pharmacological approaches are available or are currently in development. Data from a randomized, double-blind study of patients with COPD suggest that LABA/LAMA combination treatment may provide improved bronchodilator efficacy versus LABA/ICS combination treatments.51 “Triple therapy” with a LAMA, LABA, and ICS can improve pulmonary function and COPD symptoms and reduce the rates of exacerbations versus an anticholinergic alone.6,52-54 The phosphodiesterase-4 inhibitor roflumilast was US FDA-approved in 2011 as an oral medication.55 Patients with severe or very severe COPD and a history of exacerbations have fewer moderate or severe exacerbations with roflumilast versus placebo,56 regardless of concomitant use of LABAs.57 Once-daily LABAs, termed “ultra-LABAs,” such as indacaterol (US FDA-approved in 2011),58 may help improve adherence,8 although this has not been shown in clinical trials. Ultra-LABA/LAMA combinations (eg, indacaterol/glycopyrronium59) are currently in development.
Existing pharmacologic therapies for COPD do not reduce long-term declines in lung function6; there is a need for novel therapies that modify disease progression.60 Potential approaches for future COPD treatments may include agents that activate histone deacetylase-2 or inhibit phosphoinositide 3-kinase to improve sensitivity to ICS and inhibitors of p38 kinase and cathepsin C to decrease inflammation.60
COPD PHENOTYPING
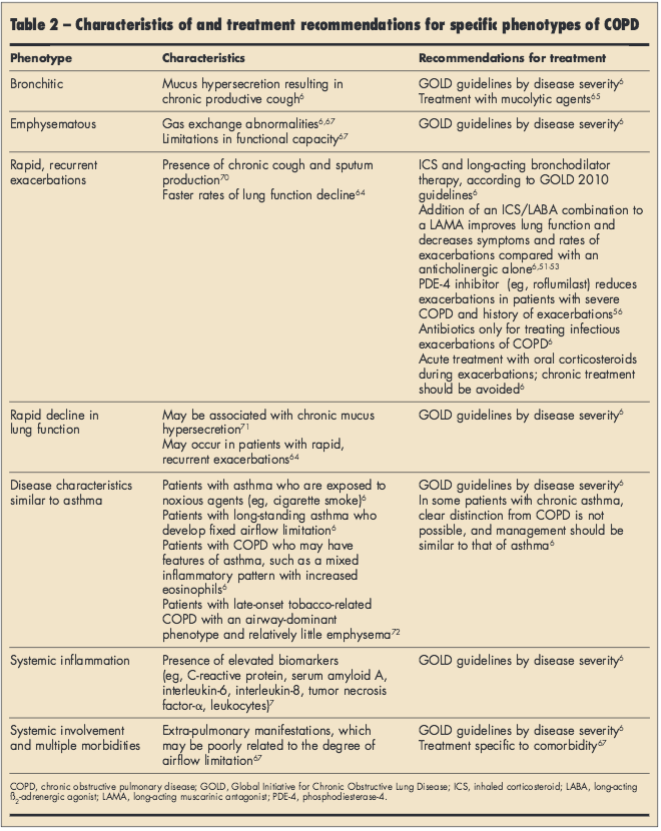
Early studies suggest that COPD clinical subpopulations, such as “frequent exacerbators,” may require more aggressive COPD management earlier in the disease.61-64 Data from the COPDGene study showed an association between chronic bronchitis and younger age, smoking, worse respiratory symptoms, and a greater history of severe exacerbations.65 These findings suggest that for patients with COPD with chronic bronchitis, reducing mucus production and smoking cessation may be extremely important. Other research is ongoing in the Subpopulations and Intermediate Outcomes Measures in COPD Study (Spiromics; http://www.cscc.unc.edu/spir/), the COPDGene Study (http://www.copdgene.org/), and the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study (http://www.eclipse-copd.com/) assessing mechanisms and identifying potential measures of disease progression. Historical phenotypes (chronic bronchitis and emphysema)66 and those that have been proposed based on clinical manifestations67-69 are described in Table 2.
CONCLUSIONS
Several pharmacological therapies are available for the treatment of COPD. Current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend treatment selection according to the patient’s disease severity.6 Within a severity category, patient phenotypes and other disease characteristics, device selection, and costs may influence the choice of therapy. Initiation and maintenance of pulmonary rehabilitation programs are important for optimal COPD management in all patients with COPD. Careful monitoring of patients during therapy is necessary to recognize disease worsening and the need for changes in treatment. Improving patient adherence and screening for comorbidities such as depression also contribute to better disease management. In the future, novel therapies may improve bronchodilator efficacy and reduce side effects compared with current treatments and modify disease progression.
Acknowledgments
The author thanks Kristen Quinn, PhD, Anny Wu, PharmD, and Cynthia Gobbel, PhD, from Scientific Connexions (Newtown, PA) who provided medical writing support funded by AstraZeneca LP (Wilmington, DE).
References:
1. Bleecker ER, Emmett A, Crater G, et al. Lung function and symptom improvement with fluticasone propionate/salmeterol and ipratropium
bromide/albuterol in COPD: response by beta-agonist reversibility. Pulm Pharmacol Ther. 2008;21:682-688.
2. Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957-965.
3. Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742-750.
4. Celli BR, Tashkin DP, Rennard SI, et al. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respir Med. 2011;105:1176-1188.
5. Prentice HA, Mannino DM, Caldwell GG, et al. Significant bronchodilator responsiveness and
“reversibility” in a population sample. COPD. 2010;7:323-330.
6. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Updated 2010. http://www.goldcopd.org. Accessed October 5, 2011.
7. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the
future of COPD. Am J Respir Crit Care Med. 2010; 182:598-604.
8. Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11:149-162.
9. Michele TM, Pinheiro S, Iyasu S. The safety of tiotropium—the FDA’s conclusions. N Engl J Med. 2010;363:1097-1099.
10. Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive
pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:1439-1450.
11. US Food and Drug Administration. Briefing information for the November 19, 2009, meeting of the Pulmonary-Allergy Drugs Advisory Committee. http://www.fda.gov. Accessed July 7, 2011.
12. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
13. Rodrigo GJ, Castro-Rodriguez JA, Nannini LJ, et al. Tiotropium and risk for fatal and nonfatal cardiovascular events in patients with chronic obstructive pulmonary disease: systematic review with meta-analysis. Respir Med. 2009;103:1421-1429.
14. Donohue JF. Therapeutic responses in asthma and COPD: bronchodilators. Chest. 2004;126:125s-137s.
15. Pauwels RA, Löfdahl CG, Laitinen LA, et al; for the European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340:1948-1953.
16. Vestbo J, Sørensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819-1823.
17. Burge PS, Calverley PMA, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297-1303.
18. Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902-1909.
19. Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332-338.
20. Singh S, Loke YK. An overview of the benefits and drawbacks of inhaled corticosteroids in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;5:189-195.
21. Halpin DM, Gray J, Edwards SJ, et al. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 201l;65:764-774.
22. Mapel D, Schum M, Yood M, et al. Pneumonia among COPD patients using inhaled corticosteroids and long-acting bronchodilators. Prim Care Respir J. 2010;19:109-117.
23. Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641-647.
24. Sin DD, Tashkin D, Zhang X, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet. 2009;374:712-719.
25. Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377:1032-1045.
26. Grimes GC, Manning JL, Patel P, et al. Medications for COPD: a review of effectiveness. Am Fam Physician. 2007;76:1141-1148.
27. Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with chronic obstructive pulmonary disease. Am J Med. 2009;112:348-355.
28. Laforest L, Denis F, Ganse EV, et al. Correlates of adherence to respiratory drugs in COPD. Prim Care Respir J. 2010;19:148-154.
29. Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50:1360-1374.
30. Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51:158-172.
31. Dolovich MB, Ahrens RC, Hess DR, et al.Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest. 2005;127:335-371.
32. Young RJ, Murphy KR. Devices for aerosol delivery in the treatment of adults with asthma or chronic obstructive pulmonary disease (COPD) in the United States. Advance for NPs & PAs. 2011. Accessed October 9, 2011.
33. Yawn BP. Translating guidelines into community practice: signs and symptoms are not enough. Prim Care Respir J. 2011;20:111-112.
34. Upton J, McCutcheon E, Loveridge C, et al. What provokes experienced COPD clinical practitioners in the UK to initiate or change medication? A consensus study. Prim Care Respir J. 2011;20:155-160.
35. Eltayara L, Becklake MR, Volta CA, et al. Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:1726-1734.
36. American Thoracic Society. Dyspnea. Mechanisms, assessments, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159:
321-340.
37. Kendrick KR, Baxi SC, Smith RM. Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26:216-222.
38. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648-654.
39. Jones PW, Chen W-H, Wilcox TK, et al. Characterizing and quantifying the symptomatic features of COPD exacerbations. Chest. 2011;139:1388-1394.
40. Leidy NK, Wilcox TK, Jones PW, et al; the EXACT-PRO Study Group. Standardizing measurement of chronic obstructive pulmonary disease
exacerbations. Am J Respir Crit Care Med. 2011;183:323-329.
41. Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:401-406.
42. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497-1505.
43. Vestbo J, Anderson JA, Calverley PMA, et al. Adherence to inhaled therapy, mortality, and hospital admission in COPD. Thorax. 2009;64:939-943.
44. Nici L, Donner C, Wouters E, et al; on behalf of the ATS/ERS Pulmonary Rehabilitation Writing Committee. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390-1413.
45. Lamers F, Jonkers CC, Bosma H, Penninx BW, Knottnerus JA, van Eijk JT. Summed score of the Patient Health Questionnaire-9 was a reliable and valid method for depression screening in chronically ill elderly patients. J Clin Epidemiol. 2008;61:679-687.
46. Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:19-38.
47. Lötters F, van Tol B, Kwakkel G, et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J. 2002;20:
570-576.
48. Tarpy SP, Celli BR. Long-term oxygen therapy. N Engl J Med. 1995;333:710-714.
49. Washko GR, Fan VS, Ramsey SD, et al; for the National Emphysema Treatment Trial Research Group. The effect of lung volume reduction surgery on chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;177:164-169.
50. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059-2073.
51. Rabe KF, Timmer W, Sagkriotis A, et al. Comparison of a combination of tiotropium plus formo-terol in moderate COPD. Chest. 2008;134:255-262.
52. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in COPD patients. Am J Respir Crit Care Med. 2009;180:741-750.
53. Singh D, Brooks J, Hagan G, et al. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63:592-598.
54. Aaron SD, Vandemheen KL, Fergusson D; for the Canadian Thoracic Society/Canadian Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545-555.
55. US Food and Drug Administration. FDA approves new drug to treat chronic obstructive pulmonary disease. http://www.fda.gov. Accessed August 1, 2011.
56. Calverley PMA, Rabe K, Goehring U-M, et al; for the M2-124 and M2-125 Study Groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
57. Bateman ED, Rabe KF, Calverley PMA, et al. Roflumilast with long-acting ß2-agonists for COPD: influence of exacerbation history. Eur Respir J. 2011;38:553-560.
58. US Food and Drug Administration. FDA approves Arcapta Neohaler to treat chronic obstructive pulmonary disease. http://www.fda.gov. Accessed July 8, 2011.
59. van Noord JA, Buhl R, LaForce C, et al. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease.
Thorax. 2010;65:1086-1091.
60. Bourbeau J, Johnson M. New and controversial therapies for chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:553-554.
61. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19:113-118.
62. Rennard SI, Farmer SG. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:88-92.
63. Tanabe N, Muro S, Hirai T, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1653-1659.
64. Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847-852.
65. Kim V, Han MK, Vance GB, et al; and the COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene study. Chest. 2011;140:626-633.
66. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:S77-S120.
67. Celli BR. Chronic obstructive pulmonarydisease phenotypes and their clinical relevance. Proc Am Thorac Soc. 2006;3:461-466.
68. Burgel P-R, Paillasseur J’L, Caillaud C, et al; on behalf of the Initiatives BPCO Scientific Committee. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J. 2010;36:531-539.
69. Cho MH, Washko GR, Hoffmann TJ, et al. Cluster analysis in severe emphysema subjects using phenotype and genotype data: an exploratory investigation. Respir Res. 2010;11:30-38.
70. Burgel P-R, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975-982.
71. Vestbo J, Prescott E, Lange P; and the Copenhagen City Heart Study Group. Association of chronic mucus secretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153:1530-1535.
72. Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest. 2004;126:117S-124S.


