Treatment of Hidradenitis Suppurativa: A Desperate Need for Comparative Studies
AUTHORS:
Mohammed D. Saleem, MD; William W. Huang, MD, MPH; and Steven R. Feldman, MD, PhD
CITATION:
Saleem MD, Huang WW, Feldman SR. Treatment of hidradenitis suppurativa: a desperate need for comparative studies. Consultant. 2016;56(12):1066-1067.
Hidradenitis suppurativa (HS), a chronic inflammatory disorder affecting the apocrine glands, is characterized by recurrent bouts of painful inflammatory nodules with subsequent sinus tract and abscess formation. On average, patients experience the development of 2 inflammatory nodules per month. Inflammatory nodules have a mean duration of 6.9 days, although 62% of persons with them may have at least one permanent tender nodule.1
HS has a significant impact on social interactions and quality of life2,3—worse than other skin disorders such as alopecia, atopic dermatitis, and facial vascular anomalies.4,5 Weight loss, smoking cessation, topical clindamycin, oral antibiotics (doxycycline, amoxicillin-clavulanate, clindamycin, rifampin, ciprofloxacin, moxifloxacin, metronidazole), antiandrogens (oral contraceptives, spironolactone, cyproterone acetate, finasteride), metformin, acitretin, zinc gluconate, infliximab, adalimumab, anakinra, ustekinumab, cyclosporine, dapsone, botulinum toxin, laser surgery, and excisional surgery have all been reported to potentially improve the signs and symptoms of HS.6
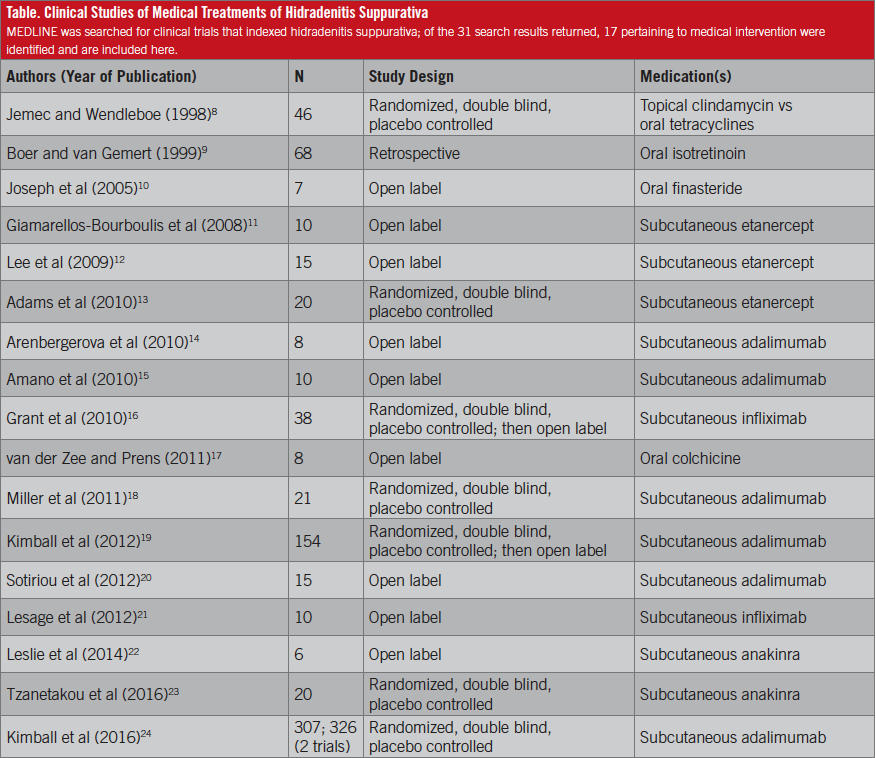
Despite the prevalence and significant impact of HS on patients’ quality of life, treatment is not standardized, and no well-defined, accepted evidence-based guidelines exist. In 1983, Clemmensen demonstrated a significant decrease in abscesses, inflammatory nodules, and pustules with the use of topical clindamycin.7 More than 30 years later in 2016, first-line treatment for stage 1 (localized) disease still includes topical clindamycin.6 Of the 17 clinical trials of medical intervention that have been indexed in MEDLINE (Table),8-24 only one compares treatment regimens: Jemec and Wendelboe8 conducted a double-blind, randomized controlled trial comparing topical clindamycin with tetracycline, and they found no differences between the 2 HS treatments.
Woodruff and colleagues proposed a treatment algorithm for HS in 2015.6 Unfortunately, many of the treatments have not been assessed in randomized controlled trials and are based on anecdotal study results. Applying evidence-based screening recommendations is much needed.
Given that the US Preventive Services Task Force25 recommends screening all children and adults for obesity and referring patients with a body mass index of 30 kg/m2 or more for intensive, multicomponent behavioral interventions, treating obesity as a first step in the HS treatment protocol seems appropriate. In addition, HS patients should be asked about tobacco use, and if applicable, the physician should advise against the use of tobacco and offer pharmacotherapy agents approved by the US Food and Drug Administration to assist.26
Because a multimodal treatment approach to HS is generally required, comparative studies are needed to guide the development of treatment protocols. Until then, the treatment of HS may continue to vary based on a physician’s professional experience, personal preference, training, comfort level, and, above all, best judgment.
Mohammed D. Saleem, MD; William W. Huang, MD, MPH; and Steven R. Feldman, MD, PhD, are in the Department of Dermatology at Wake Forest School of Medicine in Winston-Salem, North Carolina.
Disclosures:
Mohammed D. Saleem, MD, has no conflicts of interest to disclose.
William W. Huang, MD, MPH, has no conflicts of interest to disclose.
Steven R. Feldman, MD, PhD, is a consultant and speaker for Galderma, Stiefel/GlaxoSmithKline, Abbott, Warner Chilcott, Janssen, Amgen, PhotoMedex, Genentech, Biogen Idec, and Bristol-Myers Squibb. He has received grants from Galderma, Astellas, Abbott, Warner Chilcott, Janssen, Amgen, PhotoMedex, Genentech, Biogen Idec, Coria/Valeant, PharmaDerm, Ortho, Aventis, Roche Dermatology, 3M, Bristol-Myers Squibb, Stiefel/GlaxoSmithKline, Novartis, Medicis, Leo, HanAll Pharmaceutical, Celgene, Basilea, and Anacor. He has received stock options from PhotoMedex. He is the founder of and holds stock in Causa Research.
References:
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14(5):389-392.
- Onderdijk AJ, van der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2013;27(4):473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90(3):264-268.
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90(1062):216-221.
- von der Werth JM, Jemec GBE. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144(4):809-813.
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90(12):1679-1693.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22(5):325-328.
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39(6):971-974.
- Boer J, van Gemert MJP. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40(1):73-76.
- Joseph MA, Jayaseelan E, Ganapathi B, Stephen J. Hidradenitis suppurativa treated with finasteride. J Dermatolog Treat. 2005;16(2):75-78.
- Giamarellos-Bourboulis EJ, Pelekanou E, Antonopoulou A, et al. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol. 2008;158(3):567-572.
- Lee RA, Dommasch E, Treat J, et al. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2009;60(4):565-573.
- Adams DR, Yankura JA, Fogelberg AC, Anderson BE. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146(5):501-504.
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49(12):1445-1449.
- Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49(8):950-955.
- Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62(2):205-217.
- van der Zee HH, Prens EP. The anti-inflammatory drug colchicine lacks efficacy in hidradenitis suppurativa. Dermatology. 2011;223(2):169-173.
- Miller I, Lynggaard CD, Lophaven S, Zachariae C, Dufour DN, Jemec GBE. A double-blind placebo-controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol. 2011;165(2):391-398.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846-855.
- Sotiriou E, Goussi C, Lallas A, et al. A prospective open-label clinical trial of efficacy of the every week administration of adalimumab in the treatment of hidradenitis suppurativa. J Drugs Dermatol. 2012;11(5 suppl):s15-s20.
- Lesage C, Adnot-Desanlis L, Perceau G, et al. Efficacy and tolerance of prolonged infliximab treatment of moderate-to-severe forms of hidradenitis suppurativa. Eur J Dermatol. 2012;22(5):640-644.
- Leslie KS, Tripathi SV, Nguyen TV, Pauli M, Rosenblum MD. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(2):243-251.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152(1):52-59.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422-434.
- Final recommendation statement: obesity in adults: screening and management. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-adults-screening-and-management. October 2014. Accessed November 7, 2016.
- Final recommendation statement: tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. October 2015. Accessed November 7, 2016.


