A Pregnant Woman With Lax Joints and Skin
A 25-year-old woman presents for a prenatal visit. She has an unremarkable medical history except for hypermobility of the joints and a tendency to bruise easily. Her mother and sister also have very lax joints. In addition, her mother has mitral valve prolapse and has had several joint dislocations. The patient's sister had 1 pregnancy that resulted in the delivery at 32 weeks' gestation of a girl, now aged 4 years, who has increased joint laxity.
The patient has a healthy 3-year-old son and has no history of miscarriages or reproductive problems. Her husband is also healthy, and their marriage is non-consanguineous.
What is the most likely diagnosis—and what are the implications for this patient's pregnancy?
Ehlers-Danlos syndrome, classic type
Ehlers-Danlos syndrome (EDS) is a hereditary multisystem disorder caused by a variety of abnormalities in the synthesis and metabolism of collagen. The incidence is 1 in 5000 live births; 90% of cases are EDS type I, II, or III.1,2
Recognition of EDS before or during pregnancy permits monitoring for maternal cardiovascular or uterine complications and for premature delivery caused by amniotic membrane fragility.3,4 Additional risks to the mother and child include uterine rupture during delivery and complications of incision or episiotomy repair. The severity of the pregnancy complications varies with the type of EDS. Complications are likely to be mild in this patient, who has type I or II disease.1-3
The clinical spectrum of connective-tissue dysplasias
Recognition of connective-tissue dysplasia is crucial because the ocular, cardiovascular, and skeletal complications are preventable.1,2 The term “dysplasia” describes congenital abnormalities of connective-tissue growth and distinguishes this disorder from the acquired changes of autoimmune connective-tissue disease (eg, lupus or scleroderma).
The medical and family history and the physical findings suggest the diagnosis. The physical examination is paramount because DNA testing is still limited.
Patients with connective-tissue dysplasia may have a family history of skeletal problems or early deaths and a personal history of eye, cardiac, and joint disorders.
Characteristic clinical findings are listed in Table 1. Affected patients may be tall and have long limbs, with the increased arm span and decreased upper/lower segment ratio of the “marfanoid” habitus.5
| Table 1 — Clinical findings in connective-tissue dysplasia |
| • Tall, thin habitus with increased arm span and decreased upper/lower segment ratio • Long face with aged appearance and high palate that often necessitates orthodontic appliances • Eye changes, including blue sclerae, myopia, lens dislocation, glaucoma, and retinal detachment • Cardiac changes, including valvular prolapse, insufficiency, arrhythmia, and cardiomyopathy • Vascular changes, including aortic dilation and arterial ruptures that produce crises or sudden death • Pulmonary changes, including spontaneous pneumothorax, emphysema, and sleep apnea • Chest and abdominal wall changes, including pectus carinatum or excavatum and hernias • Skeletal changes, including lax joints with arthritis and dislocations, scoliosis, flat feet, and osteoporosis • Skin changes, including hyperelasticity, unusual scarring, frequent bruising, and stretch marks • Spinal changes, including dural ectasia with abdominal protrusions and atlantoaxial instability
|
Specific syndromes associated with connective-tissue dysplasia are shown in Table 2; they include Marfan syndrome (154700, the McKusick number for Marfan syndrome that allows access to online information about the disorder).5 Characteristic ocular findings are myopia attributable to a long anterior-posterior axis of the globe, flat corneas and, in 70% of patients, a lens that is displaced upward into the anterior chamber, with potential glaucoma. Slit-lamp examination can reveal the extent of dislocation, including laxity of the ciliary ligament or fluttering of the iris (iridodonesis). The full spectrum of skeletal changes listed in Table 1 may be present as well as dural ectasia, which can produce anterior myeloceles that resemble ovarian cysts.
| Table 2 — Syndromes with connective-tissue dysplasiaa |
| EDS type I classic (130000)b EDS type II classic (130010) EDS type III hypermobility (130020) EDS type IV vascular (225400) EDS type VI ocular-scoliosis (229200) EDS type VIIA, B arthrochalasis (130060) EDS type VIIC dermatosparaxis (225410) EDS type VIII periodontal (130080) Marfan syndrome (154700) Homocystinuria (236300) Beals contractural arachnodactyly (121050) Shprintzen-Goldberg syndrome (182212) Loeys-Dietz syndrome (609192) Stickler syndrome (108300) Larsen syndrome (150250) Osteogenesis imperfecta (166210) Pseudoxanthoma elasticum (264800) |
| EDS, Ehlers-Danlos syndrome. a Many other syndromes have lax connective tissue with less specific signs or symptoms. b McKusick number assigned to genetic diseases in the Online Mendelian Inheritance in Man database at www.ncbi.nlm.nih.gov/sites/entrez?db=omim. Searching on disease name, McKusick number, or symptoms yields clinical information and links to causative genes or DNA testing (www.genetests.org). The McKusick number serves as a reference for disease information. |
The Steinberg sign and the Walker sign indicate the presence of arachnodactyly and joint hyper- extensibility, respectively. The Steinberg sign is positive if the thumb protrudes beyond the ulnar border when clenched in the fist. The Walker sign is positive if the fifth finger and thumb overlap when wrapped around the opposite wrist.

Genetic mutations associated with connective-tissue dysplasias
Marfan syndrome is caused by variable mutations in the very large fibrillin gene. DNA testing may miss a mutation; thus, it can confirm but not exclude a diagnosis of Marfan syndrome.5 Diversity and overlap of pathways for fibrillin, collagen, and elastin gene function limit DNA testing for all connective-tissue dysplasias, including EDS.
Recent molecular findings in other connective-tissue syndromes have placed fibrillins within a transforming growth factor b (TGF-b)-signaling pathway, a finding that suggests new approaches to therapy. Shprintzen-Goldberg (182212)6 and the similar Loeys-Dietz syndrome (609192) have mutations in the TGF-b receptor, while Beals congenital contractural arachnodactyly (121050) involves mutation in fibrillin-2. TGF-b antagonists, such as the angiotensin receptor blocker losartan, prevent aortic aneurysms in mouse models.7 Trials are under way to determine whether losartan is more effective than atenolol for patients with vascular dilatation, an approach that could apply to other disorders within the fibrillin/TGF-b pathway.6,7
Analogous signal pathways may be defined for the collagen genes that cause Stickler syndrome (108300) or the elastin genes that cause pseudoxanthoma elasticum (264800), and these may be relevant to the diverse mutations that produce EDS. Other therapeutic clues may be derived from the vascular, thrombotic, and learning disorders in patients with homocystinuria (236300), including the homocysteine elevations found in some patients with coronary artery disease. Homocystinuria can produce a phenotype strikingly similar to that of Marfan syndrome. Some patients with homocystinuria are responsive to vitamin B6 (pyridoxine) supplementation.8
Clinical Characteristics of Ehlers-Danlos Syndrome
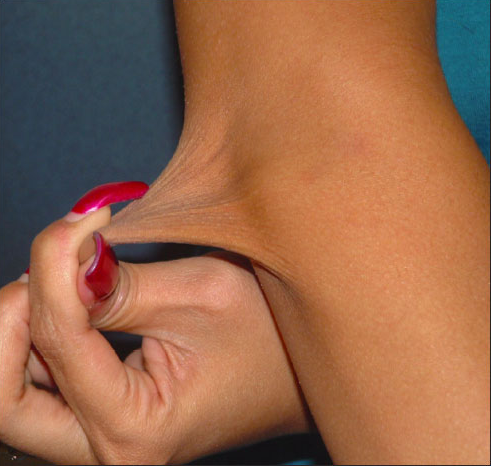
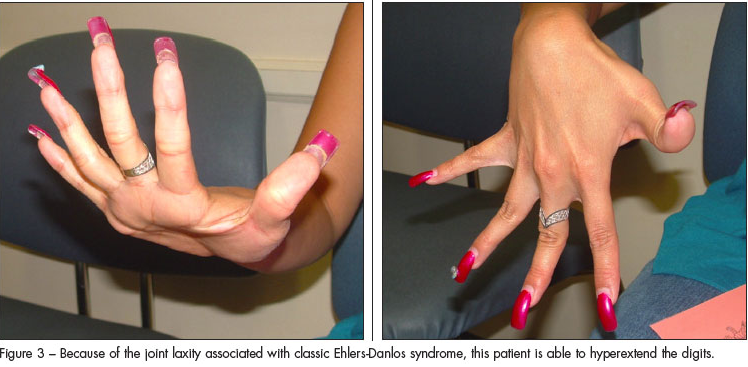
As with other connective-tissue dysplasias, the diagnosis of EDS is based on the family history, clinical symptoms, and physical findings with occasional assistance from DNA testing.1,2,8,9 Patients with the classic EDS types I and II have elastic skin (Figure 1), a tendency to bruise easily, delayed wound closure, and “cigarette paper” scarring (Figure 2). Increased joint laxity (Figure 3) can result in pain and arthritis related to activity. Tissue laxity can lead to inguinal and umbilical hernias, pectus carinatum or excavatum, kyphoscoliosis, flat feet, and ligament tears.1 Characteristic ocular features include myopia, strabismus, blue sclerae, and epicanthal folds. Easy eversion of the eyelids (Metenier sign) and the ability to touch the nose with the tongue (Gorlin sign) may be present.1 Cardiovascular risks include arterial dissection or aneurysms, valvular prolapse, and bleeding or hematomas after surgery.
The classic and rarer types of EDS involve changes in various collagen molecules, which allow preliminary diagnosis by demonstrating biochemical alterations of collagens secreted by cultured skin fibroblasts. In some cases, DNA testing can define corresponding gene defects, such as those in the collagen I alpha-1 (COL1A1) and collagen V alpha-1 or alpha-2 (COL5A1 and COL5A2) genes that are found in classic EDS.1,2,8,9
Other types of EDS include familial hypermobility (type III), vascular or “ecchymotic” (type IV), ocular-sco- liosis (type VI), arthrochalasis (type VIIA, B), dermatosparaxis (type VIIC), and periodontal (type VIII) (see Table 2). Overlap of symptoms prevents discrete separation of categories, and certain forms (such as the X-linked type V or the periodontal type VIII) have been placed in the “other” category or removed from the EDS list altogether (eg, former type IX with occipital horn syndrome that is X-linked and allelic to Menkes syndrome [309400]).9
The familial hypermobility (type III) EDS may also present with velvety, hyperextensible skin and a tendency to bruise easily. Dislocations and subluxations result in unstable joints that over time can lead to degenerative joint disease and osteoarthritis.1,2 Vascular (type IV) EDS poses grave clinical risks because of arterial aneurysms that can cause heart disease or spontaneous intestinal rupture.3 Pregnancy in these patients can be life-threatening because of these risks, in addition to the possibility of uterine rupture.4
Joint hypermobility is confined to the fingers in patients with type IV EDS, and the skin is thin and translucent. Mutations that disrupt the synthesis and structure of type III collagen (eg, COL3A1 gene mutations) have been found in type IV EDS. Less disruptive amino acid changes in type III collagen have been found in a few cases of type III EDS, although the mutation is unknown in most patients with type III EDS.1,2
Ocular-scoliosis (type VI) EDS exhibits autosomal recessive inheritance because of mutations in the PLOD1 gene. The gene encodes the lysyl hydroxylase enzyme that produces hydroxylysine cross-links in collagen. Affected patients have progressive scoliosis, brittle cornea, hyperelasticity of the skin, and hypermobility of the joints.8,9
Arthrochalasis (types VIIA and B) EDS exhibits autosomal dominant inheritance with mutations in the COL1A1 and COL1A2 genes that encode the type I collagen chains (thus overlapping with mutations in classic EDS). Patients present with stretchy, fragile skin as well as unstable and loose joints that can lead to osteoarthritis and fractures.
Dermatosparaxis (type VIIC) EDS presents similarly to the arthrochalasis type but is autosomal recessive, with mutations that disrupt procollagen N-proteinase enzyme activity (catalyzing an amino-terminal cleavage necessary for collagen chain assembly). Clinical manifestations include premature rupture of membranes; delayed closure of fontanels; growth retardation; short limbs; and characteristic facies with micrognathia and prominent, puffy eyelids.1,2,8,9
Preventive care and pregnancy-related issues
Preventive care. To avoid injury, persons with EDS should try to prevent falls and they should not participate in contact sports.1,2,8 Injuries that disrupt skin integrity require sutures placed outside and within the wound. Adhesive strips can help keep skin edges together during scar formation. Orthopedic procedures such as bracing or fusion of joints may be performed to reduce ligamentous laxity and thus prevent recurrent dislocations. Physical therapy and a regular exercise program (swimming and light weight lifting) can help forestall dislocations by strengthening muscles and stabilizing joints. Regular office visits are necessary to monitor patients for potential worsening of the disease.8
Genetic counseling is essential because EDS is associated with either an autosomal dominant inheritance with a 50% recurrence risk or an autosomal/X-linked recessive inheritance with a 25% recurrence risk.8 Further information about the genetic and pregnancy-related risks of EDS is available on Web sites such as that of the Ehlers-Danlos National Foundation (for patients) and that of the Ehlers-Danlos Support Group (for parents of patients) at www.ehlers-danlos.org.
Care during pregnancy. Pregnant women with EDS types I, II, and III are at increased risk for premature delivery, postpartum hemorrhage, and uterine or bladder prolapse; these patients should be closely monitored.3,4 High maternal mortality attributable to rupture of the aorta or uterus is associated with EDS type IV. Other complications include severe vaginal or perineal lacerations during vaginal delivery and wound dehiscence or uterine scarring after cesarean section.
Consider planned cesarean section at 32 to 34 weeks of gestation, because most spontaneous deliveries in patients with EDS occur between weeks 32 and 35. Cesarean delivery may minimize risks of arterial rupture and perineal trauma, but it can be associated with perioperative hemorrhage or wound dehiscence.3,4
In patients with EDS who have vascular disease and potential aneurysms, care during pregnancy should be similar to that for women with Marfan syndrome.
Patients who have aortic root dilation before pregnancy are at higher risk for dissection, which can be monitored by trans-esophageal echocardiography. Surgical repair of the aortic root by a composite graft has been successful in patients with Marfan syndrome and is mandatory during pregnancy if the root diameter approaches 55 mm.3-5
Multidisciplinary approach. An approach that involves specialists in cardiology, ophthalmology, orthopedics, and genetics—coordinated by the primary care physician—is required for patients with EDS or other connective-tissue dysplasias. In addition, specialists in perinatology and maternal-fetal medicine are especially needed for the woman with EDS who is contemplating pregnancy to ensure an optimal outcome.


