Pharmacologic Management of Pain in Older Patients
Introduction
We are all likely to experience pain at some point in our lives. How that pain is best managed depends on its etiology, acuity, and pathophysiology. Coexisting medical conditions and the potential risk for adverse effects play a larger role in treatment choices for older adults. In this article, we present treatment options for both acute and persistent pain. A step-wise approach with rationale for treatment choices is discussed. In addition to a variety of pharmacologic treatment options, physical therapeutics, modalities, and focused interventions are presented.
Treatment of Acute versus Persistent Pain
When considering management of pain in older adults, the choice of treatment depends on the acuity of the issue. Acute injuries or acute exacerbations of chronic conditions should be treated in a different way than persistent pain. Unless contraindicated, pharmacologic management of acute pain, including postoperative pain, should include 2-4 days of around-the-clock acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). NSAIDs can be administered in oral or topical form. If painful muscle spasms are present, a muscle relaxant should also be given. Initial doses should be administered at night in case sedation develops. If a patient is taking a chronic regimen of pain medications that includes a short-acting medication for breakthrough pain, the frequency should be increased, followed by an increase in dosage strength if required. The patient will need to be monitored for an increase in potential adverse events. Appropriate laboratory tests should be done to monitor kidney and liver function before any medication increase. If patients are taking long-term medications for pain management, including long-acting opiates, they should not be discontinued prior to any planned surgery. The surgeon and anesthesiologist should be informed of any medications being taken prior to the planned procedure. Coordination with the physician who is managing the patient’s pain should take place preoperatively to ensure that a plan is in place to allow the best postoperative pain control possible.
Nonpharmacologic methods should be included in any treatment plan for older adults with pain. Acute injuries will respond to the Rest, Ice, Compression, and Elevation, or RICE, principle; “R” stands for rest of the affected body part. Pain after an acute injury is a signal that there is tissue damage and is meant to serve a protective function. If it hurts to walk on a broken leg, you are less likely to walk on it and cause further damage. By not using an injured area, healing can occur. Immobilizing an injured area can help prevent motion through already damaged tissues. Examples of this include an elastic wrap applied to the injured area, a sling to prevent excessive shoulder motion, or an aircast for a sprained ankle. In addition, use of assistive devices such as canes or walkers can help decrease mechanical stress through an injured area. A single-point cane can unweight mechanical forces through the hip joint by up to 11%.1 A walker provides even more support and is especially helpful if a lower extremity has weight-bearing restrictions due to an acute fracture. For patients who are non–weight-bearing, a standard walker, not a rolling walker, should be utilized to decrease the risk of falls. Walkers provide more stability than axillary crutches. Training prior to the use of an assistive device will reduce the risk of injury related to improper positioning.2 Specialized footwear may alleviate chronic foot or ankle pain and also provide improved stability for gait.
Although a detailed review is beyond the scope of this article, other nonpharmacologic modalities including superficial heat or ice, transcutaneous electrical nerve stimulation (TENS) units, focused slow, sustained stretching of the affected muscle and joints, and low-level aerobic activity can also be helpful for either acute injury or persistent pain.3 An acute injury or acute exacerbation of persistent pain should be considered for a course of physical or occupational therapy to provide specialized modalities, biomechanical retraining, and instruction for a daily home exercise program for the patient.
For patients with persistent pain, the focus should be on function, including regular assessments by healthcare providers, as well as with any interventions made.4,5 If a patient’s function is not improving with a specific intervention, alternatives should be considered. If nonpharmacologic methods are not fully effective for analgesic and functional improvement, medications may be initiated. There are several existing clinical practice guidelines and review articles on this subject.6-10
Changes in metabolism that occur with aging alter the effectiveness and increase the risk for adverse events with medication usage in older patients.3 With increased age, it becomes more difficult to get optimal serum concentrations of medications and it is also more difficult to clear medications from the system. Gastrointestinal (GI) motility is decreased, and absorption by the GI system may be altered by medications or prior surgery, especially gastric bypass. An increased body mass index allows fat-soluble medications to have longer half-lives. Some aspects of liver metabolism may decrease, resulting in longer half-lives of medications. Glomerular filtration rate decreases with age, causing decreased excretion and prolonged effects of any active metabolites of the medication. Anticholinergic side effects such as constipation, urinary retention or incontinence, confusion, or even new onset of movement disorders are much more common in the elderly and may limit the use of some medications, such as tricyclic antidepressants.11
Route of administration is also important. If the oral route is being utilized for food entry, then medications for nonemergent treatment should be given orally as well. Blood concentrations achieved via oral medications are more steady as compared to intravenous dosing, thus reducing the risk of adverse effects in the older population.12
Over-the-Counter Medications
In 1990, the World Health Organization (WHO) provided initial recommendations for the treatment of cancer pain.13 These have been carried over into the treatment of chronic pain conditions. Initial treatment of nonacute, nonemergent pain with acetaminophen products and nonsteroidal analgesics is recommended. Even though many medications with these ingredients are available as over-the-counter preparations, caution must be exercised to prevent serious adverse events. The Food and Drug Administration (FDA) recently put forth a final rule requiring a “black box warning” focused on limiting the single dose limit (decreased from 1000 mg to 650 mg) and ensuring an accurate total daily intake for acetaminophen products. For products containing acetaminophen, a new labeling recommendation was made: Do not exceed the recommended dosage because severe liver damage may occur (42 FR 35346 at 35494).14 Issues specifically cited with this included the use of combination medications that contain acetaminophen with opiate medications, such as hydrocodone and oxycodone. The FDA guideline stated that these products should be clearly labeled so that consumers could more easily include the acetaminophen from these products in their total daily dosage.
Recent studies suggest that acetaminophen use is the most common cause of acute liver failure in adults in the United States.15 Most of these are related to accidental overuse. Overdoses of acetaminophen are related to a significant number of Emergency Department visits as well as events of serious liver failure every year.16,17 Although the current recommendation is for no more than 4 grams of acetaminophen products within a 24-hour period, persons with existing liver disease or those who drink more than three drinks containing alcohol daily are cautioned to reduce their intake even further. Serum liver function tests should be documented prior to initiating treatment with acetaminophen. For a new injury or acute exacerbation of a chronic problem, around-the-clock dosing for several days is likely to be more effective as compared to waiting for the pain to be uncontrolled to take a dose. Patients should be counseled to avoid alcohol while taking acetaminophen. After several days, treatment should be weaned to allow for the lowest possible dose of acetaminophen.
The other component of initial treatment recommended by WHO is for the use of NSAIDs. Older adults are at higher risk of adverse events related to NSAIDs.18 The difference in the risk:benefit ratio between an acute pain condition that is likely to be self-limited versus persistent pain is especially critical in the use of NSAIDs. Appropriate patient selection would include acute musculoskeletal injuries such as a lower back strain or shoulder tendonitis. For these patients, the lowest effective dose should be used for the shortest period of time in order to decrease the risk of adverse events. Individualized decisions to use NSAID therapy should be based on needs for pain management; risk for GI, cardiovascular, and nephrotoxicity; and potential drug interactions. The most common risks include significant GI bleeding, which is further increased with the co-administration of aspirin for cardiovascular disease.19 NSAIDs should be avoided in patients with a history of peptic ulcer disease, bleeding disorders, or if they are taking anticoagulants. The NSAID salsalate has lesser antiplatelet activity, and earlier studies indicated fewer GI effects,20 but this has not been corroborated by larger-scale clinical trials as yet. Cyclooxygenase-2 (COX-2) selective inhibitor NSAIDs, such as celecoxib, have fewer GI risks but have been shown to have increased cardiovascular toxicity.21 Patients with renal impairment or who are at risk for nephrotoxicity due to volume depletion and use of diuretics should be administered NSAIDs with caution. Close monitoring of their renal function is indicated while taking NSAID therapy.
A four-pronged approach to risk stratification of NSAID use is proposed.10 Ibuprofen or naproxen are good first-line choices for patients with low GI risk. If the GI risk is higher, an NSAID can be co-administered with either misoprostol or a proton pump inhibitor such as omeprazole, esomeprazole, or pantoprazole. If a COX-2 inhibitor is chosen due to higher GI risk, co-administration of low-dose aspirin for cardioprotection should be considered. For patients with significant risk of both GI and cardiovascular toxicity, and for whom NSAID is considered the best therapeutic option, the combination of either naproxen or celecoxib with low-dose aspirin therapy should be considered.
Topical NSAIDs (eg, diclofenac) have been effective for short-term use in pain relief. Although they have produced fewer GI adverse effects related to oral NSAIDs,22 caution is still advised for patients with significant risk of GI bleed or who are on concomitant anticoagulation therapy.23 Longer-term studies are not yet available. Local skin reaction to the adhesive components of the patch formulation and the inability to maintain full skin contact (ie, falling off) are the primary complaints of patients when using these medications. Capsaicin is a topical treatment derived from pepper plants. It binds to the TRPV1 receptors that are normally activated by excessive heat. Presynaptic supply of substance P, an excitatory neurotransmitter, is depleted by the prolonged exposure of these neurons to capsaicin.24 Patients need to be instructed on how to carefully apply this treatment to avoid exposure to sensitive mucous membranes.
Muscle Relaxants
The term muscle relaxant is used for a diverse category of medications. This includes medications that directly affect neurotransmitters within the central nervous system (CNS), peripheral-acting agents at the level of the muscle itself, and also medications whose exact mechanisms are unknown but are generally thought to act as CNS depressants. Muscle relaxants are most often used for acute injury involving muscle pain, such as with strain or sprain, but with appropriate patient selection can be a useful adjunct for persistent pain as well. Some understanding of the physiology of neuromuscular transmission is necessary to understand how these medications work. Afferent, or sensory, impulses are carried from the periphery to the spinal cord, where interneurons synapse to both ascending and descending efferent, or motor, nerves. Alterations in the system, including decreased central inhibition or increased ectopic firing of nerves, can affect pain signal transmission.25
Spasticity is a velocity-dependant increase in muscle tone. It occurs when the central inhibition of the reflex arc from afferent muscle spindles is diminished. This is seen with an upper motor neuron lesion, such as stroke, Parkinson’s disease, or multiple sclerosis. This presynaptic inhibition is mediated by several neurotransmitters. Gamma-aminobutyric acid (GABA) has two distinct receptors. The GABA-A receptor exists primarily in ligand-gated chloride channels at interneurons in the spinal cord. When these channels are activated, hyperpolarization of the type Ia afferent nerve terminals occurs, and there is less neurotransmitter released to signal motor neurons to fire. GABA-A receptors provide most of the rapid inhibition in the CNS. The GABA-B receptor is fewer in number and believed to help regulate ion channels. There are three centrally-acting antispasticity medications: diazepam; baclofen; and tizanidine. Dantrolene and botulinum toxins act on the peripheral nervous system to alter the efferent pathway to muscle spasticity.
Release of GABA and subsequent binding to GABA-A receptors in the spinal cord is facilitated by the benzodiazepine diazepam. The side effects, including sedation and decreased muscle strength, usually limit its usefulness in older patients for persistent pain. Baclofen is a GABA-B receptor agonist that acts in the spinal cord and the CNS. It suppresses the release of excitatory neurotransmitters, essentially providing increased central inhibition. At higher doses, baclofen can also cause lethargy. Implanted intrathecal pumps can deliver the baclofen intrathecally, limiting the dose and sedation experienced. This indication is for severe generalized spasticity and not in use for pain management. Presynaptic motor neuron inhibition is increased by tizanidine, a centrally-acting alpha-2 agonist (see Table I for comparison and dosages).
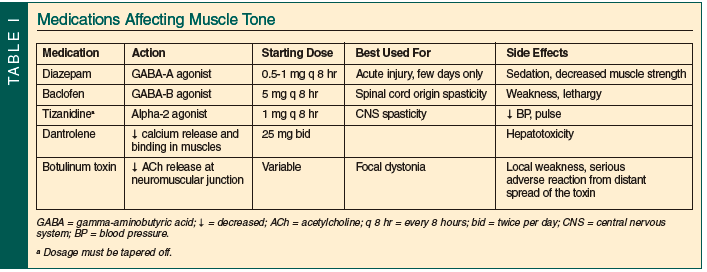
Botulinum toxins are purified neurotoxins that are injected directly into the muscles themselves. The toxins inhibit the release of acetylcholine at the neuromuscular junction. The effect of this is to stop the signal between the nerve and muscle itself, essentially paralyzing the area of muscle affected by the neurotoxin. There are currently three botulinum toxins approved for use in the United States: onabotulinumtoxinA; abobotulinumtoxinA; and rimabotulinumtoxinB. All three are FDA-approved for cervical dystonia, while onabotulinumtoxinA also is approved for strabismus, blepharospasm, and, most recently, upper-extremity spasticity as seen after a stroke. Research is ongoing regarding a primary pain relief component from the botulinum toxin that would be separate from its neuromuscular blockade.26-28 The most severe adverse reactions to these medications are from distant spread of the toxin. The injections can be done only once every 3 months to avoid development of antibodies against this biologic substance. Cost of the medication and insurance coverage also limit its current usage for pain management, but for refractory cases it should be considered in consultation with a physician trained in its usage.
Acute localized areas of muscle spasm are diagnosed by tenderness, asymmetry of muscle size, restriction of range of motion, and palpation of taut bands within the muscle itself. Palpation of these areas may also refer pain to other body regions, such as seen with palpation of the teres minor muscle in the posterior axillary fold, producing dysesthesias into the fourth and fifth digits. These muscular trigger points may mimic radiculopathy or other nerve entrapments. Electrodiagnostic studies can be helpful in distinguishing the etiology of these types of symptoms. For short-term treatment of a condition that includes areas of acute muscle spasm, medications that produce muscle relaxation through an unclear mechanism of CNS depression can be considered with caution. Cyclobenzaprine, carisoprodol, and methocarbamol all share the potential side effects of drowsiness and dizziness. Studies on long-term use, especially in older adults, have not been done. For most older patients with persistent pain, the risks outweigh the benefits of these types of medications.
Opiates
According to the WHO analgesic ladder, opiates are indicated to treat both acute and chronic pain that is moderate-to-severe in intensity.29 It was traditionally thought that opiates are more beneficial in the treatment of nociceptive pain, but it is commonly used to treat all types of pain, including neuropathic and mixed nociceptive-neuropathic pain syndromes. Opiates have been extensively studied in cancer pain, but it has been demonstrated that opiates can improve pain and function in nonmalignant pain.30 It is also common to use opiates in combination with other drugs to treat pain, such as NSAIDS, anticonvulsants, and antidepressants. When using opiates in the geriatric patient, it is important to start at the lowest dose, titrate carefully, and assess the patient frequently for side effects.
There are two general classes of opiate drugs: agonist and agonist-antagonist drugs. Opiate agonist medications work directly on the mu receptor at the level of the dorsal horn of the spinal cord and at the brain to achieve analgesia. In reality, opiate receptors are found throughout the body and may work in a variety of ways to block pain. Agonist-antagonist opiates are combination drugs that have an opiate antagonist such as naloxone built into the product. The antagonist only becomes active with manipulation of the product, the benefit being that it becomes more tamper-resistant. Opiates can be administered in the oral, intravenous, intramusclular, transdermal, and transbuccal forms. They are available as short-acting (2 and 4 hr) and extended-release (12, 24, and 72 hr) duration of activity.
The gold standard of opiate medications is morphine sulfate. Generally, morphine is the opiate to which all others are compared. Morphine is available in both extended-release and immediate-release formulations. Morphine is relatively easy to titrate and can be used in both acute and chronic pain. Two studies demonstrate that elderly patients have a greater sensitivity to the potency of morphine and a decreased ability to clear morphine when compared to younger patients.31,32 This equates to increased analgesia and a prolonged analgesic effect when compared to younger patients given the same dose and administration. Morphine also has neurotoxic metabolites that are renally excreted. In patients with even mild renal involvement, these metabolites can accumulate and have significant side effects in the elderly, such as myoclonus, delirium, hallucinations, and significant sedation. When initiating morphine in the elderly patient, it is important to start at low doses and titrate slowly. It is wise to consider all elderly patients as having some element of renal slowing when considering morphine as an agent. It is prudent to check renal function prior to initiating morphine sulfate.
Oxycodone is available in both immediate- and extended-released formulations. It is considered 1.5-2 times the potency of morphine. This drug is metabolized several times through the cytochrome P450 (CYP450) isoenzyme 2D6 pathways. Oxymorphone and noroxymorphone are its active metabolites.33 Until recently, it was believed that the pharmacokinetics of oxycodone vary little in the adult population, and that inhibition of the CYP450 isoenzyme 2D6 does not significantly impair pain scores.34,35 However, concomitant use of oxycodone with CYP450 3A4 inhibitors such as macrolide antibiotics, azole antifungals, and protease inhibitors may result in increased oxycodone levels. When an elderly patient may require a CYP450 3A4 inhibitor, some thought should be given to titrate down the oxycodone or to very closely monitor the patient for adverse events.
Codeine, a drug many falsely consider to be a weak opiate, has six active metabolites, the most potent metabolite being morphine.36 Similarly, hydrocodone is metabolized to its very potent active form of hydromorphone via the CYP450 isoenzyme pathway.33 These drugs will be affected by drugs that inhibit the P450 isoenzmye 2D6 pathway. Hydromorphone, used as a single agent or as a metabolite, is five times more potent than morphine, and caution should be used when titrating in the elderly.
Transdermal fentanyl can be extremely useful in a select group of elderly patients. The transdermal delivery system provides analgesia to patients who have swallowing difficulty or who prefer to not take oral medications. It can also provide analgesia for up to 72 hours. However, the drug has a slow onset of action, making it difficult to use in the patient with acute pain, and titration becomes longer and more difficult. The drug is heavily protein-bound, making it a less effective drug to use in the cachectic, frail patient. The absorption of the drug can increase as body temperature increases, being problematic with sick, febrile patients. Once the patch is removed, the time to drug elimination is significantly longer in the elderly patient.37
Methadone is a potent, long-acting opiate analgesic that may have some added benefits in neuropathic pain syndromes because it can be an antagonist to the N-methyl-D-aspartate receptors.38 However, methadone should be used with extreme caution in the elderly patient. Methadone is extensively metabolized by the liver and GI mucosa. Both the half-life of the drug and the elimination of methadone vary in individuals, especially the elderly. Drug interactions occur with both inducers and inhibitors of the CYP450 isoenzyme 2D6 system.39,40 For these reasons, when using methadone in the elderly patient, use small doses and titrate slowly. Methadone should always be considered a long-acting opiate, even though the duration of activity as an analgesic may be relatively short. The practice of combining methadone with other long-acting opiates should be avoided in the elderly patient.
Oxymorphone is a powerful semisynthetic opiate analgesic available in both an extended-release and immediate-release formulation. It is considered to be about three times more potent than morphine sulfate. In elderly patients, plasma concentrations increase up to 40% when compared with younger patients.41 Here, caution should be used in converting to oxymorphone and titrating the drug in the elderly patient.
Accumulation of active metabolites with increased risk for adverse events leads to the recommendation that meperidine and propoxyphene not be utilized by elderly patients. Meperidine is a relatively weak opioid agonist with a shorter duration of action as compared to morphine. However, normeperidine, which is formed by the liver metabolism of meperidine, can be neurotoxic, including the development of seizures. This is especially problematic in patients with renal impairment whose excretion of the drug is decreased.42 Propoxyphene should not be given to the elderly patient. The drug has minimal analgesic properties beyond aspirin. Its active metabolite, norpropoxyphene, is associated with cardiac and CNS toxicity, leading to increased mortality and morbidities in the elderly patient.43
When initiating opiate therapy, selection of the opiate is critically important. Opiate-naïve patients require small initial doses. In chronic pain (ie, pain lasting > 3 mo), an extended-release formulation should be considered. The benefits of a long-acting agent are many. As drug-dosing burden goes down, compliance with taking the medication goes up. Extended-release formulations provide 24-hour pain control, enabling the patient to remain out of pain, improving his/her overall quality of life. Patients taking extended-release formulations may require short-acting opiates for breakthrough pain.
Proper pain management requires finding the balance between optimal analgesia and side effects.44 The most common side effects in opiate use are constipation, respiratory depression, sedation, pruritus, nausea and vomiting, myoclonus, depression, and delirium. Most side effects are fairly mild and disappear with removal of the opiate therapy. Most patients will develop tolerance to the side effect when on chronic therapy. McNichol et al45 looked at 67 opiate clinical trials and concluded that there are diverse interventions for the management of opioid-related side effects, but more studies and guidelines need to be established. In clinical practice, opiate side effects should be anticipated, especially constipation and pruritis, and a treatment plan should be initiated with the onset of opiate therapy.
Persistent Neuropathic Pain
There are several reviews of trials performed on the treatment of persistent primarily neuropathic pain.46-48 The pharmacologic treatment plans for these patients will differ from the acute pain treatments listed above, but should still include nonpharmacologic options. The treatments of choice for persistent pain include antidepressants, membrane stabilizer medications, and possibly opiates. Patients treated only with opiates for persistent neuropathic pain have higher incidences of adverse events, including the development of opiate hyperalgesia. The combined effects of two types of medications may lead to better analgesia with fewer side effects. Research on this purposeful polypharmacy is being done with two different classes of medications given simultaneously or in one of the newer combination medications such as tapentadol, which includes an opiate agonist and a norepinephrine uptake inhibitor.
Antidepressants
Depression and anxiety commonly accompany persistent pain. Patients with persistent pain should also be screened for depression and its related symptoms. Antidepressant medications can help improve mood, sleep, and, although it is considered an “off-label” use, have also been shown to have independent analgesic properties.49 Antidepressant medications being used for pain treatment should be started at low doses in older persons, typically no more than half of the recommended dosage. Dosages should be increased gradually over the first month of treatment. Dosages for effective pain relief are usually less than those for depression. Careful monitoring for adverse events is important, as they may occur with increased frequency in the older population. Careful patient education with regard to the independent analgesic properties of these medications helps patients understand that you are not treating them “just for depression,” and can improve compliance. Although they are not in extensive clinical use any longer, monoamine oxidase inhibitors should not be combined with any other antidepressants due to the risk of significant adverse events.
The first antidepressants used for pain were the tricyclic antidepressants (TCAs). They act through inhibition of reuptake of the neurotransmitters serotonin and norepinephrine. (See Table II for a comparison of the different antidepressants.) However, unwanted side effects due to the nonspecific inactivation of histamine, acetylcholine, and alpha-1 adrenergic receptors also can occur. Common side effects include sedation, dry mouth, constipation, urinary retention, sinus tachycardia, memory impairment, orthostatic hypotension, blurred vision, and weight gain. TCA selection is usually determined by the side-effect profile. Nortriptyline is generally thought to have the lowest anticholinergic side-effect profile.50 An electrocardiogram should be documented prior to any TCA therapy, as these medications can prolong the QT interval on electrocardiogram and worsen cardiac arrhythmias. Other medications that affect the CYP450 system in the liver may alter serum concentrations of TCAs. Abrupt discontinuation of TCAs can result in a withdrawal syndrome including fever, sweating, nausea, or dizziness. Careful adherence to instructions for dosing of these medications is important, as an amount that is three to five times the usual dose can be toxic. Symptoms of toxicity include seizures, coma, and cardiac arrhythmias.

Selective serotonin reuptake inhibitors (SSRIs) are the most widely prescribed antidepressant medications. This is most likely due to their efficacy and low side-effect profile. However, the role for SSRIs in pain management separate from depression has not been proven effective.51
The newer classes of antidepressants, serotonin-norepinephrine reuptake inhibitors (SNRIs), have the analgesic efficacy of TCAs with a better side-effect profile.52 This is due to the lack of alpha-1, cholinergic, or histaminergic receptor activation by these medications. Duloxetine and venlafaxine are both FDA-approved for depression. Duloxetine also has indications for the treatment of pain related to diabetic peripheral polyneuropathy and fibromyalgia. Both drugs can lower seizure thresholds and have a “black box” warning for increased suicidality in children, adolescents, and young adults.
Treatment of a coexisting anxiety disorder is a bit more problematic due to the potential drug-drug interactions of anxiolytics and pain medications. Benzodiazepines work by binding to the inhibitory neurotransmitter GABA. Benzodiazepines can cause sedation, confusion, respiratory depression, and, in the elderly, increased agitation and disinhibition. In combination with opioid pain medications, the risks increase for potential overdose. Short-acting benzodiazepines such as alprazolam and lorazepam have a rapid onset of action (roughly 15 min). Their short half-lives of 2-3 hours can result in rebound of anxiety, creating a roller coaster effect. Longer-acting benzodiazepines such as clonazepam and diazepam may avoid the ups and downs but are rarely indicated as a first-line treatment in older patients due to higher risk of side effects. Buspirone is a serotonin agonist without addictive properties and less likelihood of causing cognitive impairment. A treatment course of several weeks is required with buspirone, so it is not an effective agent for immediate anxiety relief. Any long-term use of anxiolytics should be done with the patient under the ongoing supervision of a behavioral healthcare professional.
Anticonvulsants
Neuropathic pain, which is as a result of abnormal signals from the nervous system, is likely to respond better to anticonvulsant medications than pure nociceptive pain. It is thought that ectopic firing of neurons contributes to neuropathic pain, including trigeminal neuralgia, postherpetic neuralgia, and diabetic peripheral polyneuropathy. “Misfiring” of both sodium and calcium channels in the nerve cell membranes are involved in the generation of these action potentials. By stabilizing the overactive nerve cell membrane and decreasing the ectopic action potentials, neuropathic pain can be decreased.53 Because of the potential for more serious adverse events, the medications referred to as membrane stabilizers were usually considered for patients who cannot achieve adequate analgesia with other medications or combination of medications.54 However, there are a few medications in this class with good safety profiles, even in older adults55 (Table III).

Gabapentin and pregabalin inhibit the release of excitatory neurotransmitters glutamate, norepinephrine, and substance P through binding at the voltage-gated calcium channels.56 Gabapentin has been in use longer and appears to have an overall better safety profile in older patients.46 This may be related to its larger dosage range, which allows a wider range of dosing to be given. However, this also means that the upward titration of gabapentin usually takes longer to achieve a clinical result.47 Both medications require reduced dosing with a reduced creatinine clearance.
Anticonvulsant medications including carbamazepine, oxcarbazepine, lamotrigine, valproic acid, and topiramate all cause sodium channel blockade. Each agent has unique side effects. Carbamazepine may cause agranulocytosis or aplastic anemia. Complete blood counts should be followed regularly for patients on this medication. Hyponatremia may develop with oxcarbazepine, while a rash is the most common side effect of lamotrigine. (See Table III for comparison of dosages and the most serious adverse events.) A direct comparison of this class of medications has not yet been done to compare efficacy. A slow titration upwards of the medication with the lowest apparent side-effect profile is the best initial option when adding this class of adjuvant medications. Since it is likely to affect long-term compliance, insurance coverage and the patient’s ability to cover the copayments may also be included in the initial selection decision. If an agent in this class in not effective and switching to another medication is planned, documentation of the dosages and lack of response or adverse events that occurred may be necessary to obtain authorization for coverage of a nonformulary drug.
Topical lidocaine in a 5% patch is approved for postherpetic neuralgia. Postherpetic neuralgia is a persistant allodynia at the site of a herpes zoster rash, or shingles. Both the occurrence of shingles and the subsequent development of postherpetic neuralgia are more common in the elderly population with poorer response to treatment.57 Lidocaine inhibits sodium ion channels, stabilizing the neuronal membrane and impeding conduction of the action potential.58 Up to three patches can be applied simultaneously for a total of 12 out of 24 hours. All patches need to be removed for a period of 12 hours to avoid toxicity. In contrast to topical opioid patches, these patches may be cut and placed on affected areas. The patches should not be applied to areas of inflamed or broken skin, as this may affect their absorption and risk for toxicity. Patients with elevated body temperatures will also have increased absorption of the medication and should be carefully monitored for toxicity including tinnitus and ataxia. Topical lidocaine patches should not be used with superficial heat such as heating pads, as this increases absorption and the risk of adverse events.59 As with other topical agents, the most common reaction is local skin irritation or lack of good skin adherence. For postherpetic neuralgia, a better choice may be prevention with a high-potency single dose vaccine that has been shown to reduce the incidence of postherpetic neuralgia by 67%.60
Interventional Pain Management
For the past few decades, the management of pain has been evolving as new advances in technology develop. Inventions such as the modern fluoroscope, ultrasound, computed tomography, and magnetic resonance imaging have allowed physicians to gain a real-time image of the internal anatomy of the body, which previously was only possible in cadavers. By looking at the anatomy in real time, practitioners are now able to guide instruments such as needles or catheters into the body and place them into specific locations with amazing accuracy. This new-found ability has opened up a novel avenue for the diagnosis and treatment of pain and has spawned a relatively new medical subspecialty called interventional pain management. Below is a brief discussion of selected procedures within interventional pain management, including a description of the procedures, indications, and evidence-based recommendations as outlined in the 2009 American Society of Interventional Pain Physicians (ASIPP) guidelines.61
Facet Joint Intervention
The facet joints (or zygapophyseal joints) are synovial planar joints that connect one vertebra to the next sequential vertebra. The joint capsule contains nociceptive fibers, making it a potential pain generator. Because the facet is a synovial joint, it is also prone to osteoarthritis and spurring. The prevalence of facet-mediated pain ranges from approximately 15% to 45% in the lumbar spine, and approximately 30% to 40% in the cervical spine.62 The three main interventions to the facet joint are: (1) intra-articular joint injection with anesthetics and/or corticosteroids; (2) blockade of the medial branch of the dorsal ramus, which supplies the facet joint with anesthetics and/or corticosteroids; and (3) ablation of the medial branch either chemically or with radio waves. All three interventions are indicated for facet-mediated pain in the cervical, thoracic, or lumbar spine. However, evidence for intra-articular injections into the facet joint is limited or negative, whereas evidence for medial branch blockade and medial branch ablation is moderate-to-strong for both short- and long-term relief of cervical and lumbar facet pain.
Epidural Injections
The epidural space houses the exiting nerve roots of the spinal cord, and is a sturdy area for depositing medication. There are three different approaches for accessing the epidural space, and once arrived, typically a local anesthetic and/or corticosteroid is injected. The first method is a caudal block, which is only applicable to the lumbar spine. A needle is inserted into the sacral hiatus, and advanced into the epidural space. This approach has limited scope and can only affect the cauda equina and lower segments. The second method is an interlaminar approach, which can be done at any level of the spine. In this case, the needle is placed midline between the spinous processes of two adjacent vertebrae and slowly advanced into the epidural space. This approach is the most common one utilized. The final method is a transforaminal approach, where a needle is inserted laterally and obliquely and passed directly into the epidural space within the neural foramen of a specific nerve root, mainly in the lumbar spine. This approach is the most technically difficult but also requires the least amount of injectant. Epidural injections are indicated in radicular pain conditions such as herniated disc disease, sciatica, or spinal stenosis. Evidence for caudal blocks was strong for relief of lumbar pain, as well as for post-laminectomy and lumbar spinal stenosis patients. The interlaminar approach had moderate-to-strong evidence for short-term and long-term relief of cervical radiculopathy. For lumbar radiculopathy, the evidence was moderate-to-strong for short-term relief and weak for long-term relief. The transforaminal approach yielded strong evidence for both short- and long-term relief of lumbar radiculopathy.61
Spinal Cord Stimulators
The use of electrical stimulation to the spinal cord for analgesic purposes first began in the 1960s, with the work of Shealy et al.63 Since that time, the process has become more refined to the point that now several commercial vendors produce versions of implantable stimulator kits. Spinal cord stimulation requires three components: (1) a lead wire, which is inserted into the epidural space adjacent to the desired spinal segments; (2) an electric pulse generator, which is embedded under the skin and connected to the lead wire; and (3) an external remote control system by which to control and recharge the pulse generator.64 The system works according to the gate control theory, which states that by flooding the dorsal column of the spinal cord with electrical information, it prevents nociceptive signals from being transmitted to the brain and processed as pain.65 Spinal cord stimulators are currently indicated for use in failed back syndrome, as well as complex regional pain syndrome types 1 and 2. The evidence for spinal cord stimulators is moderate-to-strong for long-term pain relief.
Intrathecal Drug Delivery
For patients with chronic, refractory, malignant, or nonmalignant pain, an option to consider is an implanted intrathecal pump. In this procedure, a catheter is surgically inserted into the intrathecal space, with the free end connected to a combination pump and reservoir that is implanted under the skin. The pump can either operate autonomously at a constant rate or be programmable with an external programming device. The reservoir can then be filled with medication, which will slowly be delivered directly to the cerebrospinal fluid. The main advantage of intrathecal delivery is that dosing is significantly lower than via the oral route, sparing the patient from many of the systemic side effects of the medication. Options for medications to use within the pump include morphine, hydromorphone, or fentanyl for analgesia, baclofen for spasticity, clonidine for neuropathic pain, and several others. Medications may also be used in combination to achieve multiple goals.66 Evidence for the use of intrathecal pumps is moderate for long-term relief of chronic noncancer pain.
Summary
Based on changes in physiology, a higher percentage of medical comorbidities, and a higher incidence of adverse events as we age, treating older adults with pain is a more complex issue as compared to treating younger persons. Each of these factors needs to be considered when designing a treatment plan uniquely tailored to an individual. Changes in liver and kidney function, in addition to the risk for GI and cardiovascular events, may determine treatment choices to a certain extent. While a short course of treatment for an acute issue may not differ greatly from younger patients, except in dosages of medications used, persistent pain in older patients demands careful assessment and an ongoing monitoring program to determine the efficacy of the interventions being used. In addition to determining the level of analgesia being produced, adverse events also need to be predicted and prevented if at all possible to allow patients to continue on their medication regimens. By selecting the appropriate combination of medications and nonpharmacologic interventions, clinicians can help manage their patients’ pain and improve the quality of their lives at any age.
Dr. Jermyn has received speaker honoraria from Pfizer.
The other authors report no relevant financial relationships.
Dr. Janora is Associate Professor, Dr. Jermyn is Associate Professor and Chair Designate, and Dr. Surve is Assistant Professor, Division of Rehabilitation Medicine, NeuroMusculoskeletal Institute, University of Medicine and Dentistry of New Jersey, School of Osteopathic Medicine, Stratford. Dr. Surve is also from the Department of Osteopathic Manipulative Medicine.
References
1. Aragaki DR, Nasmyth MC, Schultz SC, et al. Immediate effects of contralateral and ipsilateral cane use on normal adult gait. PM R 2009;1(3):208-213. Published Online: December 27, 2008.
2. Faruqui SR, Jaeblon T. Ambulatory assistive devices in orthopaedics: Uses and modifications. J Am Acad Orthop Surg 2010;18(1):41-50.
3. Clinical practice guideline for the management of postoperative pain. Veterans Health Administration/Department of Defense. www.healthquality.va.gov/pop/pop_fulltext.pdf. Accessed August 3, 2010.
4. British Pain Society and British Geriatrics Society Guidance. The assessment of pain in older people. http://www.bgs.org.uk/Publications/Publication%20Downloads/Sep2007PainAssessment.pdf. Accessed August 2, 2010.
5. Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003;106 (3):337-345.
6. American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 5th ed. Glenview, IL: American Pain Society; 2003.
7. Agency for Healthcare Research and Quality U.S. Department of Health and Human Services. Choosing nonopioid analgesics for osteoarthritis: Clinician summary guide. J Pain Palliat Care Pharmacother 2009;23(4):433-457.
8. Miaskowski C, Bair M, Chou R, et al. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 6th ed. Glenview, IL: American Pain Society; 2008.
9. Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society recommendations for improving the quality of acute and cancer pain management. American Pain Society Quality of Care Task Force. Arch Intern Med 2005;165:1574-1580.
10. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009;57:1331-1346. Published Online: July 2, 2009.
11. Barber JB, Gibson SJ. Treatment of chronic non-malignant pain in the elderly: Safety considerations. Drug Sa 2009;32:457-474.
12. Herr K, Bjoro K, Steffensmeier J, Rakel B. Acute pain management in older adults. U.S. Department of Health & Human Services. AHRQ. www.guideline.gov/content.aspx?id=10198. Accessed August 3, 2010.
13. Schug SA, Zech D, Dorr U. Cancer pain management according to WHO analgesic guidelines. J Pain Symptom Manage 1990;5(1):27-32.
14. Organ-specific warnings; Internal analgesic, antipyretic, and antirheumatic drug products for over the-counter human use; Final Monograph. Federal Register 2009;74(124):31177.
15. Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol 2007;102(11):2459-2463.
16. Watkins PB, Kaplowitz N, Slattery N, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: A randomized controlled trial. JAMA 2006;296:87-93.
17. Larson AM, Polson J, Fontana R, et al; Acute Liver Failure Study Group. Acetaminophen- induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology2005;42:1364-1372.
18. Franceschi M, Scarcelli C, Niro V, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: A prospective study of 1756 patients. Drug Saf 2008;31(6):545-556.
19. Information for healthcare professionals: Concomitant use of ibuprofen and aspirin. U.S Dept of Health and Human Services, U.S. Food and Drug Administration. Updated September 29, 2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm125222.htm. Accessed August 3, 2010.
20. Cryer B, Goldschmiedt M, Redfern JS, Feldman M. Comparison of salsalate and aspirin on mucosal injury and gastroduodenal mucosal prostaglandins. Gastroenterology1990;99:1616-1621.
21. Setakis E, Leufkens HG, van Staa TP. Changes in the characteristics of patients prescribed selective cyclooxygenase 2 inhibitors after the 2004 withdrawal of rofecoxib. Arthritis Rheum 2008;59:1105-1111.
22. Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev 2010;(6):CD007402.
23. Zacher J, Altman R, Bellamy N, et al. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin 2008;24(4):925-950. Published Online: February 14, 2008.
24. Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997;389 (6653):816-824.
25. Melen O. Muscle relaxants. In: Benzon H, Raja SN, Molloy RE, et al, eds. Essentials of Pain Medicine and Regional Anesthesia 2nd ed. Churchill Livingstone, Philadelphia, PA; 2005:159-165.
26. Tugnoli V, Capone, JG, Eleopra R, et al. Botulinum toxin type A reduces capsaicin-evoked pain and neurogenic vasodilatation in human skin. Pain 2007;130(1-2):76-83. Published Online: December 27, 2006.
27. Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain [published correction appears in Ann Neurol 2009;65(3):359]. Ann Neurol 2008;64(3):274-284.
28. Yuan RY, Sheu JJ, Yu JM, et al. Botulinum toxin for diabetic neuropathic pain: A randomized double-blind crossover trial. Neurology 2009;72(17):1473-1478. Published Online: February 25, 2009.
29. WHO’s pain ladder. World Health Organization. http://www.who.int/cancer/palliative/painladder/en/ Accessed August 3, 2010.
30. Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. CMAJ 2006;174:1589-1594.
31. Owen JA, Sitar DS, Berger L, et al. Age-related morphine kinetics. Clin Pharmacol Ther 1983;34:364-368.
32. Baillie SP, Bateman DN, Coates PE, Woodhouse KW. Age and the pharmacokinetics of morphine. Age Ageing 1989;18:258-262.
33. Coller JK, Christrup LL, Somogyi AA. Role of active metabolites in the use of opioids. Eur J Clin Pharmacol 2009;65:121-139. Published Online: October 29, 2008.
34. Kaiko RF, Benziger DP, Fitzmartin RD, et al. Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther 1996;59:52-61.
35. Guay DR. Opioid analgesics for persistent pain in the older patient. Aging Health 2006;2:669-690.
36. Facts and comparisons. Wolters Kluwer Health Website. www.factsandcomparisons.com/online-products.aspx. Accessed August 3, 2010.
37. Esteve M, Levron JC, Flaisler B, et al. Does aging modify pharmacokinetics of transdermal fentanyl? Anesthesiology 1991;75(suppl):A705.
38. Hewitt DJ. The use of NMDA-receptor antagonists in the treatment of chronic pain. Clin J Pain 2000;16(2 suppl):573-579.
39. Li Y, Kantelip JP, Gerritsen-van Schieveen P, Davani S. Interindividual variability of methadone response: Impact of genetic polymorphism. Mol Diagn Ther 2008;12:109-124.
40. Guay DR. Methadone for persistent pain in the older patient. Aging Health 2006;2:313-324.
41. Guay DR. Use of oral oxymorphone in the elderly. Consult Pharm 2007;22:417-430.
42. Mahajan G, Fishman SM. Major opioids in pain management. In: Benzon HT, Raja SN, Molloy RE, et al, eds. Essentials of Pain Medicine and Regional Anesthesia. 2nd ed. Philadelphia, PA: Churchill Livingstone; 2005:94-105.
43. Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in the older adults: results of U.S. consensus panel of experts [published correction appears in Arch Intern Med 2004;164(3):298]. Arch Intern Med 2003;163(22):2716-2724.
44. Portenoy RK. Management of common opioid side effects during long-term therapy of cancer pain. Ann Acad Med Singapore 1994;23:160-170.
45. McNichol E, Horowicz-Mehler N, Fisk RA, et al; American Pain Society. Management of opioids side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain2003;4(5):231-256.
46. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: Evidence based recommendations. Pain 2007;132(3):237-251. Published Online: October 24, 2007.
47. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clinic Proc 2010;85(3 suppl):S3-S14.
48. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: An overview of recent guidelines. Am J Med 2009;122(10 suppl 1):S22-S32.
49. Onghena P, Van Houdenhove B. Antidepressant-induced analgesia in chronic non-malignant pain: A meta-analysis of 39 placebo controlled studies. Pain 1992;49:205-219.
50. Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol 2007;151(6):737-748. Published Online: April 30, 2007.
51. Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Evidence-based data from animal and human experimental studies on pain relief with antidepressants: A structured review. Pain Med 2000;1:310-316.
52. Pernia A, Micó JA, Calderón E, Torres LM. Venlafexine for the treatment of neuropathic pain. J Pain Symptom Manage 2000;19:408-410.
53. Baron R. Peripheral neuropathic pain: From mechanisms to symptoms. Clin J Pain 2000;16(2 suppl):S12-S20.
54. Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2009 revision [published online ahead of print April 9, 2010]. Eur J Neurol.
55. Sommer BR, Fenn HH, Ketter TA. Safety and efficacy of anticonvulsants in elderly patients with psychiatric disorders: Oxcarbazepine, topiramate and gabapentin. Expert Opin Drug Saf 2007;6(2):133-145.
56. Taylor CP. The biology and pharmacology of calcium channel alpha2-delta proteins. Pfizer Satellite Symposium to the 2003 Society for Neuroscience meeting, Sheraton New Orleans Hotel, New Orleans, LA. November 10, 2003. CNS Drug Rev 2004;10:183-188.
57. Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010;9(3 Suppl):21-26.
58. Galer BS, Rowbotham MC, Perander J, Friedman E. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: Results of an enriched enrollment study. Pain 1999;80:533-538.
59. Shemirani N, Tang D, Friedland DR. Acute auditory and vestibular symptoms associated with heat and transdermal lidocaine. Clin J Pain 2010;26(1):58-59.
60. Sanford M, Keating GM. Zoster vaccine (Zostavax): A review of its use in preventing herpes zoster and postherpetic neuralgia in older adults. Drugs Aging 2010;27(2):159-176.
61. Manchikanti L, Boswell MV, Singh V, et al; ASIPP-IPM. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009;12:699-802.
62. Manchikanti L, Manchikanti KN, Cash KA, et al. Age-related prevalence of facet-joint involvement in chronic neck and low back pain. Pain Physician 2008;11(1):67-75.
63. Shealy CN, Mortimer JT, Resnick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg 1967;46:489-491.
64. Kunnumpurath S, Srinivasagopalan R, Vadivelu N. Spinal cord stimulation: Principles of past, present and future practice: A review. J Clin Monit Comput 2009;23:333-339.
65. Melzack R, Wall PD. Pain mechanisms: A new theory. Science 1965;150:971-979.
66. Belverud S, Mogilner A, Schulder M. Intrathecal pumps. Neurotherapeutics 2008;5(1):114-122.


