Peer Reviewed
Immunoglobulin G4-Related Hepatic Abscess With Comorbid Primary Hepatic Tuberculosis
Authors:
Sithu Lin, MD; Kyawzaw Lin, MD; Aung Naing Lin, MD; Thinzar Lin, MD; and Anjali Bakshi, MD
Citation:
Lin S, Lin K, Lin AN, Lin T, Bakshi A. Immunoglobulin G4-related hepatic abscess with comorbid primary hepatic tuberculosis. Consultant. 2017;57(10):604-606.
A 61-year-old woman with distant history of latent tuberculosis (TB) that had been treated with isoniazid a decade ago presented with a 1-week history of intermittent low-grade fever with associated nausea, vomiting, diarrhea, and right upper abdominal pain. She also reported having had malaise, myalgia, and headache without neck stiffness. She denied cough, dyspnea, chest pain, hemoptysis, rash, joint pain, joint stiffness, joint swelling, jaundice, abdominal pain, night sweats, and weight change.
History and Physical Examination
The patient did not smoke, drink alcohol, or use any immunosuppressive medications or illicit drugs. She recently had begun experiencing hot flashes, for which she had started taking black cohosh. She had no history of allergy or childhood asthma.
She had no family history of autoimmune diseases. She had a brother who had died from a brain lesion with underlying AIDS. She reported having visited Turkey and Sicily 4 months prior to the onset of fever. Findings of other systemic reviews were unrevealing.
At presentation, she was found to have a temperature of 38.4°C. Physical examination was unrevealing except for mild discomfort at the right upper quadrant of the abdomen, without the Murphy sign or costovertebral angle tenderness.
Diagnostic Tests
Results of a complete blood cell count showed leukocytosis with neutrophils predominant. The erythrocyte sedimentation rate was elevated at 200 mm/h (reference range, 0-29 mm/h), and the C-reactive protein level was elevated and 372 mg/L (reference range, < 3.0 mg/L). A hepatic function panel yielded the following values: total bilirubin, 1.9 mg/dL (reference range, 0.3-1.2 mg/dL), with a direct bilirubin of 1.2 mg/dL (reference range, 0.1-0.3 mg/dL); aspartate aminotransferase, 150 U/L (reference range, 10-40 U/L), alanine aminotransferase, 48 U/L (reference range, 7-56 U/L), and alkaline phosphatase, 229 U/L (reference range, 44-147 U/L). Test results for hepatitis A, B, and C were negative for acute or chronic infection. Test results for HIV infection also were negative.
Chest radiographs showed no evidence of pulmonary disease. Ultrasonography of the liver was performed, the results of which revealed a 7.6-cm heterogeneous mass in the dome of the liver, as well as cholelithiasis without evidence of cholecystitis. A subsequent computed tomography (CT) scan of the abdomen and pelvis (Figure 1) showed a multiloculated cystic lesion in the right lobe of the liver measuring 8 × 9 × 8 cm, with septations and mild hepatomegaly. CT findings also were reported as suspicious for the presence of hepatic vein thrombosis.

Figure 1: A CT scan of the abdomen and pelvis demonstrating a hepatic abscess (arrow).
Subsequent fluoroscopy-guided aspiration and multiple biopsies of the liver lesion were performed. A thick, red, sanguineous aspirate was obtained, fluid cytology results of which showed no malignant cells. The histopathology report of the biopsy samples revealed focal steatosis, portal tract inflammation, fibrosis and ductular reaction of the hepatocytes without evidence of granuloma, and abundant lymphoplasmacytic cell infiltration. Immunochemical staining showed a marked increase in IgG4-positive cells (reaching 100/high-power field), which met all the criteria for immunoglobulin G4-related disease (IgG4-RD). In addition, there was evidence of plasma-cell infiltration, with markedly increased positive IgG4 cells on immunostaining (Figure 2). Aspirated fluid cultures grew Staphylococcus aureus, and acid-fast bacilli (AFB) stain was negative while AFB culture results were pending.

Figure 2: Histologic features of IgG4-related liver abscess. Left, hematoxylin-eosin stain (magni cation ×400) showing plasma cells (arrows), and right, immunostain (magni cation ×200) showing IgG4-positive plasma cells (arrows).
Further investigations showed the serum total IgG level to be 2409 mg/dL (reference range, 694-1618 mg/dL) with a high IgG4 level of 266 mg/dL (reference range, 90-180 mg/dL). Entamoeba histolytica and Echinococcus antibodies were not detected in fluid or serum. Additional testing for tumor markers such as carcinoembryonic antigen, cancer antigen 19-9, and α-fetoprotein were unrevealing, and results of tests for autoantibodies including rheumatoid factor, antinuclear antibodies, anticyclic citrullinated peptide, anti–double-stranded DNA, anti-Smith, anti-Ro and anti-La, anti-Scl-70, antineutrophil cytoplasmic antibodies, myeloperoxidase, and proteinase-3 also were negative.
Management, Outcome, and Follow-Up
The patient was initially managed empirically with intravenous ceftriaxone and metronidazole and intravenous anticoagulation, since all clinical parameters were highly in suggestive of pyogenic liver abscess. Clinical and laboratory improvement was achieved with intravenous antibiotics and aspiration, as evidenced by the decreasing size of the abscess on repeated sonography and CT imaging. The patient was discharged home on a continuing regimen of antibiotics for a total of 4 weeks.
Six weeks after discharge, fluid AFB cultures grew Mycobacterium tuberculosis. The standard 4-drug regimen of isoniazid, pyrazinamide, ethambutol, and rifampin was initiated for 2 months, and then continued with isoniazid and rifampin for 6 to 8 months after triple-phase hepatobiliary CT scans showed the abscess to be significantly reduced in size (Figure 3). Neither glucocorticoids nor rituximab were administered due to coexisting TB. The patient remained asymptomatic at 6 months and again at 12 months after having completed the anti-TB medication regimen.
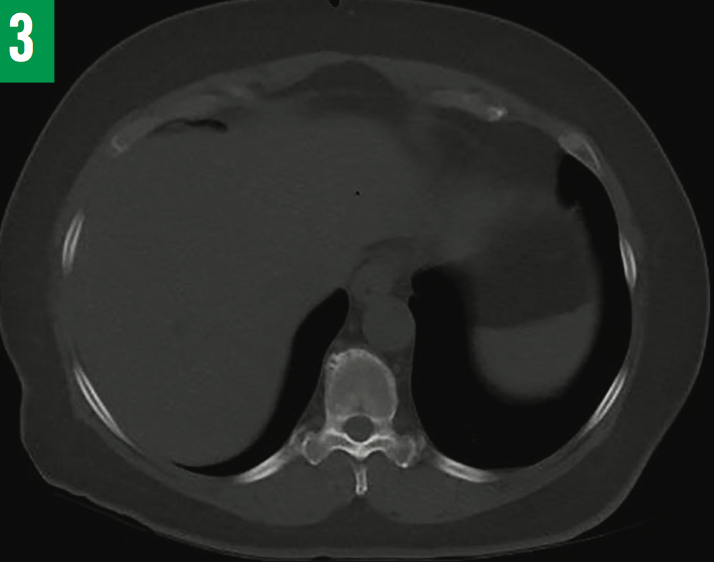
Figure 3: Repeated CT scan of the liver showing complete resolution of abscess after 6 months of anti-TB treatment.
Discussion
IgG4-RD is a relatively newly recognized systemic autoimmune spectrum disorder involving single or multiple organs and often mimicking malignancy. It is more common in middle-aged men (compared with classic autoimmune diseases, which have a female predilection) with an estimated male-to-female ratio of 3 to 1. The mean age of patients at diagnosis is 60 years. No familial or genetic susceptibility has been correlated.
The exact etiology of IgG4-RD is still elusive, but the condition is thought to result from genetic molecular mimicry following bacterial infection and/or autoimmunity.1-5 Overexpression of interleukins 4, 5, 10, and 13 and transforming growth factor β and infiltration of inflammatory cells progress to fibrosis and deposition of immune complexes, resulting in organ inflammation and dysfunction.6-11
Although the majority of reported cases have originated in Asia—particularly Japan, Korea, and China—there is no geographic difference or racial or ethnic preponderance. Autoimmune pancreatitis was the first identified IgG4-RD in 2001,12 and the pancreas is the most commonly affected organ, with an estimated 2.3 cases per 100,000 population. IgG4-related manifestations in the salivary glands and the lungs were described in 200413; to date, nearly 40 organs have been reported to be involved in IgG4-RD. Three cardinal histopathologic features—proliferative lymphoplasmacytic infiltration with IgG4-positive plasma cells, storiform fibrosis, and obliterative phlebitis—are similar across involved organs.1,14-16 Histopathology testing is the diagnostic gold standard recommended by the international pathology community; assessment of serum IgG4 concentration is useful for screening but not sufficient for diagnosis.15-18
The optimal treatment of IgG4-RD has not yet been established, but corticosteroids have been used anecdotally; rituximab alone or in combination with a corticosteroid has been shown to have better outcomes.6,7,16,17 Corticosteroid-sparing agents such as azathioprine, mycophenolate mofetil, methotrexate, cyclophosphamide, fludarabine, and bortezomib have been described as alternatives.1-3,17 In very few asymptomatic patients, self-limitation of IgG4-RD has been observed without treatment.
Correlation between TB and IgG4-RD is extremely rare, and only 3 confirmed cases have been reported in the literature. One case report documented a diagnosis of IgG4-related sclerosing sialadenitis and dacryoadenitis after treatment for cervical lymph node TB.19 In another case report, a patient developed IgG4-RD after treatment for urinary tract TB.20 The third report was a case of IgG4-related pulmonary and salivary disease coexisting with pulmonary TB.21 An additional case of IgG4-related lung disease mimicking TB recently was reported.22 However, IgG4-related hepatic abscess in association with primary hepatic TB has not been reported previously.
Our patient’s clinical presentation of fever and right-sided abdominal pain and the results of initial investigations, including CT, favored a diagnosis of hepatic abscess. Further autoantibody testing related to suspicion of hepatic vein thrombosis revealed an elevated serum IgG4 level. Histologic findings of numerous IgG4-positive plasma cells confirmed the diagnosis IgG4-RD.
The growth of M tuberculosis in AFB cultures from liver aspirate resulted in a dilemma. An absence of granulomatous histology findings ruled out other inflammatory diseases such as TB and sarcoidosis mimicking IgG4-RD. However, negative findings on chest radiographs, negative interferon-γ release assay results, and 3 consecutive negative sputum AFB culture results ruled out secondary hepatic TB and supported the final diagnosis of IgG4-related liver disease coexisting with primary hepatic TB. Complete resolution of clinical symptoms was observed after antibiotics followed by standard anti-TB medications. We did not start corticosteroids or other immunosuppressive agents because of the patient’s underlying TB.
Conclusion
If primary hepatic TB itself is a rarity, coexisting IgG4-RD with primary hepatic TB is rarer still and to our knowledge has not been previously reported. After careful review of our case and all available case reports in the literature, we believe that there are links between IgG4-RD and M tuberculosis infection. One study reported a possible association of IL-32 with chronic inflammation such as TB, and that IL-32 can be used as a predictive factor for drug-free remission in IgG4-RD.23 However, more research is required to prove the correlation between M tuberculosis infection and IgG4-RD and to establish the management options.
A high IgG4 level has been reported in hyporesponsive and asymptomatic cases of human lymphatic filariasis and onchocerciasis, along with a high level of the complement-fixing antibodies IgG1, IgG2, and IgG3, but in patients with asymptomatic cases, a high level of IgG4 and IL-10 are linked to high worm loads.24 An elevated plasma IgG4 level has also been reported in 2 patients with pulmonary Paragonimus westermani infection, a parasitic pleuropulmonary infection endemic to Asia and caused by eating raw crustaceans or wild boar meat, and the serum IgG4 level can be potentially useful as a marker of P westermani infection.25
Sithu Lin, MD; Kyawzaw Lin, MD; Aung Naing Lin, MD; and Thinzar Lin, MD, are in the Internal Medicine Department, and Anjali Bakshi, MD, is an attending physician in the Infectious Diseases Department and the associate program director of the Internal Medicine Residency Program, all at The Brooklyn Hospital Center, Affiliate of Mount Sinai Hospital/Icahn School of Medicine at Mount Sinai, Brooklyn, New York.
References:
- Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366(6):539-551.
- Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38(10):982-984.
- Pieringer H, Parzer I, Wöhrer A, Reis P, Oppl B, Zwerina J; Vasculitis and Orphan Diseases Working Group of the Austrian Society of Rheumatology. IgG4-related disease: an orphan disease with many faces. Orphanet J Rare Dis. 2014;9:110.
- Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460-1471.
- Vasaitis L. IgG4-related disease: a relatively new concept for clinicians. Eur J Intern Med. 2016;27:1-9.
- Ghably JG, Borthwick T, O’Neil TJ, Youngberg GA, Datta AA, Krishnaswamy G. IgG4-related disease: a primer on diagnosis and management. Ann Allergy Asthma Immunol. 2015;114(6):447-454.
- Islam AD, Selmi C, Datta-Mitra A, et al. The changing faces of IgG4-related disease: clinical manifestations and pathogenesis. Autoimmun Rev. 2015;14(10):914-922.
- Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
- Rispens T, Ooievaar-De Heer P, Vermeulen E, Schuurman J, van der Neut Kolfschoten M, Aalberse RC. Human IgG4 binds to IgG4 and conformationally altered IgG1 via Fc-Fc interactions. J Immunol. 2009;182(7):4275-4281.
- Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118(3):573-581.
- Zen Y, Fujii T, Harada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45(6):1538-1546.
- Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344(10):732-738.
- Taniguchi T, Ko M, Seko S, et al. Interstitial pneumonia associated with autoimmune pancreatitis. Gut. 2004;53(5):770-771.
- Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181-1192.
- Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22(1):21-30.
- Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 2015;74(1):14-18.
- Stone JH. IgG4-related disease: nomenclature, clinical features, and treatment. Semin Diagn Pathol. 2012;29(4):177-190.
- Deshpande V, Khosroshahi A. Diagnostic guidelines for IgG4-related disease with a focus on histopathological criteria. Diagn Histopathol. 2013;19(4):119-127.
- Kawano M, Yamada K, Kakuchi Y, et al. A case of immunoglobulin G4-related chronic sclerosing sialadenitis and dacryoadenitis associated with tuberculosis. Mod Rheumatol. 2009;19(1):87-90.
- Imai T, Yumura W, Takemoto F, et al. A case of IgG4-related tubulointerstitial nephritis with left hydronephrosis after a remission of urinary tract tuberculosis. Rheumatol Int. 2013;33(8):2141-2144.
- Bajema KL, Daniel JC, Hussain S, Dworkin RJ. IgG4-related pulmonary and salivary disease associated with pulmonary tuberculosis. Ann Am Thorac Soc. 2014;11(7):1165-1167.
- Tan H, Li H, Hu Y, Niu R, Pan P, Hu C. A case of solely lung-involved IgG4-related disease mimicking tuberculosis. Heart Lung. 2015;44(2):161-164.
- Shimizu Y, Yamamoto M, Yajima H, et al. Role of interleukin-32 in the mechanism of chronic inflammation in IgG4-related disease and as a predictive biomarker for drug-free remission. Mod Rheumatol. 2016;26(3):391-397.
- Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol. 2010;104(6):455-464.
- Saeki S, Horio Y, Hirosako S, Ichiyasu H, Fujii K, Kohrogi H. Elevated serum IgG4 levels in two cases of paragonimiasis. Respirol Case Rep. 2015;3(3):92-94.


