Diagnosing Asthma-COPD Overlap Syndrome
A new patient with a history of hay fever and past cigarette use presents with wheezing, a productive cough, and dyspnea. Is the correct diagnosis asthma or COPD?
This question plagues clinicians daily because dogma dictates that a patient has either asthma or COPD—but not both. However, separating asthma from COPD in clinical practice is difficult due to the overlapping features common to both diseases. The pitfall is to presume that both conditions can not possisbly exist in the same patient.
______________________________________________________________________________________________________________________________________________________
RELATED CONTENT
FDA Approves Olodaterol Inhalation Spray for COPD
Noncardioselective Beta-Blocker Use in Patients With Asthma
______________________________________________________________________________________________________________________________________________________
The Asthma-COPD Overlap Syndrome (ACOS) is a newly recognized diagnosis,1-3 one that has long been neglected in part because clinical trials for decades have consistently excluded patients with overlapping asthma and COPD, using strict inclusion and exclusion criteria. These criteria routinely excluded asthma patients from COPD studies, and COPD patients from asthma studies (Table 1). As a result there are no evidenced-based guidelines for the diagnosis and treatment of ACOS that are based on actual clinical trials of subjects with ACOS.

Definitions
A consensus ACOS description for clinical use has recently been published by both the Global Initiative for Asthma (GINA) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in 2014,3 however, a clear pharmacotherapeutic approach to these patients remains elusive. ACOS accounts for approximately 15% to 55% of patients with diseases of chronic airflow limitation.3 This syndrome represents an important population with worse outcomes than asthma or COPD alone.4
Patients with ACOS have the combined risk factors of smoking and atopy, and are generally younger than patients with COPD.1,4 ACOS patients have acute exacerbations with higher frequency and greater severity than lone COPD,4 manifest more air-trapping, and require more healthcare visits,5 despite a lower burden of cigarette smoking.
We have proposed a syndromic approach to recognize the considerable heterogeneity in obstructive airway diseases—ie, asthma [IgE or non-IgE mediated disease] and COPD should be considered as syndromes, and ACOS consists of similar phenotypes with characteristic, but nonspecific symptoms.2,3 COPD is a syndrome that includes patients with chronic bronchitis or patients with emphysema phenotypes that are united by the most common risk factor: tobacco cigarette smoking.
Based on our personal clinical experience in patients with overlapping features of asthma and COPD, ACOS can be defined as 1 of 2 clinical phenotypes.1,2
1.Asthma with partially reversible airflow obstruction—ie, based on change in FEV1 with bronchodilators, with or without emphysema or reduced carbon monoxide diffusing capacity (carbon monoxide diffusion [DLCO], <80% predicted).
2.COPD with emphysema accompanied by reversible or partially reversible airflow obstruction, with or without environmental allergies (eg, elevated total IgE or eosinophils) or reduced DLCO.
Table 2 compares the 3 syndromes of obstructive airway diseases.3,6,7

Diagnosing ACOS
The approach proposed by GINA is simple and practical.3
1. Ask if the patient has chronic airways obstruction, which can only be confirmed by spirometry.
2. Correlate history (eg, hay fever or tobacco smoking history), physical examination (eg, sinusitis, wheezing, or rhonchi), spirometry (essential to confirm airways obstruction), chest x-ray, and screening questionnaires (eg, Asthma Control Test or COPD Assessment Test).
3. Use the GINA 5-step algorithm for diagnosis and initial treatment (Table 3). The GINA alogrithm recommends assembling the clinical features that favor a diagnosis of asthma or COPD, followed by comparing the number of features in favor of asthma or COPD.
4. Evaluate the level of certainty around the diagnosis of asthma or COPD versus whether presenting features suggest ACOS as a diagnosis.
Table 3. Syndromic Approach to Chronic Airways Diseases3
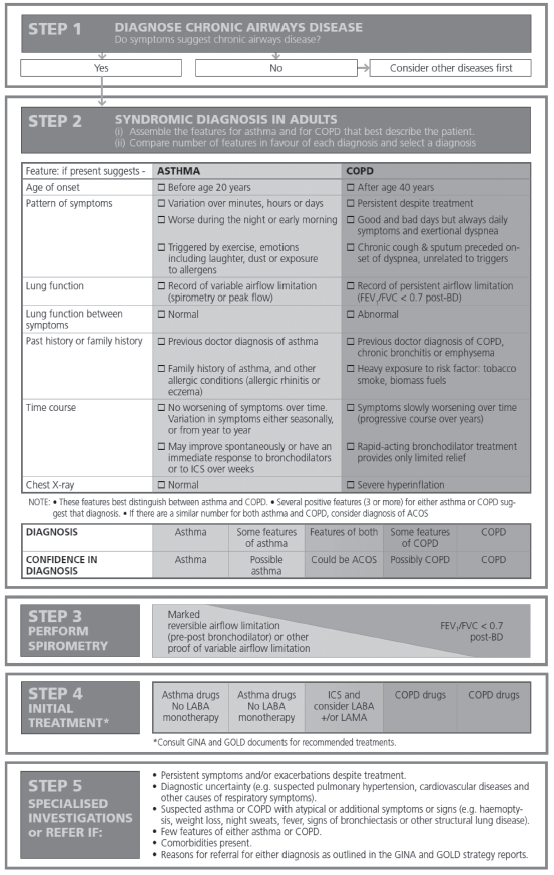
GINA reports that the prevalence rates of ACOS are between 15% and 55%, with variation by gender and age.3 In our practice, we reported a prevalence between 15% and 25% (Figures 1 and 2).1,2
Despite their relatively younger age and a lower burden of cigarette smoking, the healthcare costs for patients with ACOS is significantly greater than for COPD alone.2,3,5 Given the increasing costs of healthcare, our limited resources, and relatively high prevalence of ACOS, the time has come to better define this syndrome in order to pursue randomized clinical trials that are designed to evaluate targeted drug therapies.

Figures 1 and 2. Prevalence of obstructive airways disease in a general pulmonary clinic and in the UC Davis Medical Center
severe asthma clinics.1
Initial Therapy
The goals of treatment in all diseases of chronic airflow limitation (in lieu of a cure) are to control symptoms and prevent exacerbations, thereby reducing morbidity and mortality. Morbidity can result from coughing, wheezing, sputum production, and dyspnea on exertion. Mortality can result from frequent exacerbations and complications, including adverse drug reactions, and respiratory failure. Control is a valid concept in all diseases of chronic airflow obstruction and this is achieved through the reduction of risk and impairment (Figure 3).
The objectives or the steps needed to reduce risk and impairment, as well as prevent exacerbations, should be attainable (timeline of 3 months), measurable (eg, control questionnaire scores, FEV1, or fractional exhaled nitric oxide [FeNO]), and cost-effective (eg, number needed to treat and acceptable risk of adverse drug effects).
Current drug treatments have been incorporated into clinical practice guidelines— the 2007 NAEPP-EPR3,6 2014 GINA8 for asthma, and 2014 GOLD treatment guidelines for COPD7—all of which recognize the enduring risk of acute exacerbations. Knowledge of these treatment guidelines is important to help patients achieve basic control of their disease. The value of emerging peer-reviewed, large, randomized clinical trials and meta-analyses is that they add to current therapeutic strategies. Clinical trials that enroll patients from the real world instead of professional study subjects, are of greater value because they more closely reflect real clinical practice.
Current controller drugs for asthma action plans include inhaled corticosteroids (ICS); long-acting ß2 agonists (LABA), leukotriene receptor antagonists (LTRA); omalizumab; and theophylline. Of note, LABAs are contraindicated as monotherapy in the United States for the treatment of asthma. Current rescue drugs for asthma include short-acting ß2 agonists (SABA), short-acting muscarinic antagonists (SAMA), systemic corticosteroids, and in some cases, antibiotics.
Current maintenance or controller treatments for COPD action plans include tiotropium, a long-acing muscarinic antagonist (LAMA), LABA, LABA + ICS, LAMA + LABA, the phosphodiesterase-4 inhibitor roflumilast, theophylline, and “triple therapy” which combines LAMA + LABA + ICS. Current rescue drugs for COPD include SABA, SAMA, systemic corticosteroids, and antibiotics.
GINA recommends starting treatment for asthma first when faced with ACOS, which means the differential diagnosis is equally balanced between asthma and COPD. This recognizes the pivotal role ICS play in preventing acute exacerbations in both asthma and COPD, and even death.2,3 ICS should be prescribed and patient response assessed in 2 to 6 weeks. Failure to improve lung function (FEV1 >5% over baseline spirometry) and symptoms should raise suspicion that the patient is a nonresponder to ICS.2,9 One can consider increasing the ICS dose and/or adding a LABA or LTRA to ICS treatment. Adding theohylline and/or omalizumab may also be an option for select patients. For instance, omalizumab can only be added if the asthma component is IgE-mediated and other anti-inflammatory drugs have failed to control symptoms and exacerbations.
A LABA remains the preferred add-on to ICS in asthma but is contraindicated as monotherapy in the United States per the FDA. Experience and expertise is required with other asthma treatments (eg, LTRA and omalizumab).
Bronchodilators, such as LAMA and LABA, are first-line therapies alone or in combination in COPD. A history of acute COPD exacerbation in the past 12 months should prompt the addition of a moderate dose ICS and/or roflumilast. ICS should not be prescribed alone in COPD or in ACOS if COPD features are very pronounced. The high doses of ICS typically used in asthma are associated with a higher incidence of pneumonia in patients with COPD.
A comprehensive program for all diseases of chronic airflow limitation must address, if relevant, the complex physiological and behavioral processes that contribute to nicotine addiction and relapse. It is a major pitfall to not offer smoking cessation education and pharmacological aides repeatedly to each patient. Concurrently, additional therapies—eg, personal hygiene education, vaccinations, pulmonary rehabilitation, and the recently-added bronchial thermoplasty procedure—should be employed to control symptoms and prevent acute exacerbations where appropriate. Immunotherapy against detected aeroallergens is considered adjunctive care by the NIH-NAEPP but may have utility in select difficult-to-control cases.2
Pharmacotherapeutic considerations in ACOS must currently rely on a combination of reasoned clinical experience and logical extrapolation from the existing literature in asthma and COPD. However, until clinical trials are accomplished specifically in patients with ACOS, it would be a serious pitfall not to conduct a clinical trial in every patient to tailor therapy, ie 1 drug therapy at a time to determine effective treatments while avoiding adverse effects.

Figure 3. Pharmacotherapeutic targets in Asthma-COPD Overlap Syndrome.2,10
To Avoid Pulmonary Pitfalls in ACOS:
Do not presume that features of asthma and COPD cannot exist in the same patient.
Remember advancing age, atopy, and tobacco smoking are risk factors for ACOS.
Confirm the presence of airflow limitation using spirometry in asthma and COPD patients. Spirometry is essential to diagnose and monitor response to treatment and management.
Offer smoking cessation education and pharmacological aides to every current smoker.
Conduct a clinical trial of 1 in every patient, using 1 drug therapy (or other intervention) at a time.
Treat the component of asthma first with ICSs and ascertain objectively if lung function and symptoms improve after 6 weeks. Then consider adding long-acting bronchodilators, such as a LAMA and/or LABA.
Alert patients of the FDA’s black box warning assigned to the class of LABA in everyone, not just asthma patients. LABAs remain the preferred add-on drug to ICS.
Consider omalizumab and bronchial thermoplasty after 3 months of confirmed adherence to action plans without improvement in symptoms and/or the ability to control exacerbation risk.
Consider roflumilast early to reduce the risk of frequent acute exacerbations.
Offer pulmonary rehabilitation early to improve quality of life and reinforce patient education. ■
Samuel Louie, MD, is a professor of medicine in the division of pulmonary, critical care, and sleep medicine, department of internal medicine at the University of California, Davis School of Medicine.
Amir A. Zeki, MD, MAS, is an assistant professor of medicine in the division of pulmonary, critical care, and sleep medicine, department of internal medicine at the University of California, Davis School of Medicine.
References:
1. Zeki AA, Schivo M, Chan A, et al. The Asthma-COPD Overlap Syndrome: a common clinical problem in the elderly. J Allergy (Cairo). 2011:2011:861926.
2. Louie S, Zeki AA, Schivo M et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6(2):197-219.
3. Global Initiative for Asthma. Diagnosis of diseases of chronic airflow limitation: asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS). 2014. Accessed July 1, 2014.
4. Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127.
5. Rhee CK, Yoon HK, Yoo KH, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD. 2014;11(2):163-170
6. National Heart, Lung and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed July 1, 2014.
7. Global Initiative for Chronic Obstructive Lung Disease. Guidelines: global strategy for diagnosis, management, and prevention of COPD. 2014. Accessed July 1, 2014.
8. Global Initiative for Asthma. Guidelines: global strategy for asthma management and prevention. 2014. Accessed July 1, 2014.
9. Martin RJ, Szefler SJ, King TS, et al. The predicting response to inhaled corticosteroid efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119(1):73-80.
10.Bousquet J, Jeffrey PK, Busse WB, et al. Asthma: from bronchoconstriction to airway remodeling. Am J Respir Crit Care Med, 2000;61:1720-1745.


