Apical Hypertrophic Cardiomyopathy
Case 1. A 51-year-old man with a past medical history significant for hypertension and hyperlipidemia was admitted for evaluation of chest pain.
Diagnostic tests. Electrocardiography (ECG) findings (Figure 1) were notable for left ventricular hypertrophy (LVH) with distinct secondary T-wave changes, especially T-wave inversion in the inferolateral leads; these findings were largely unchanged from the results of an ECG obtained 8 years earlier. Cardiac marker test results were negative.
The patient then underwent a nuclear medicine treadmill stress test; he was able to perform 12 minutes on a Bruce protocol without reproduction of chest pain. Perfusion imaging results demonstrated a small area of probable mild distal inferior reversible ischemia.

An echocardiogram showed moderate LVH with an apical hypertrophic cardiomyopathy (aHCM) pattern (Figures 2A and 2B). Cardiac catheterization revealed severe diffuse disease of the right coronary artery. Ventriculography revealed a spade-like left ventricular cavity (Figures 3A and 3B), an abnormal finding compared with the appearance of the left ventricule (LV) cavity of a patient without aHCM (Figures 4A and 4B). The patient was diagnosed with paroxysmal atrial fibrillation, had been nonadherent with warfarin therapy, and later in follow-up was found to have had a cardiovascular accident (CVA) involving the middle cerebral artery territory due to a cardioembolic source.

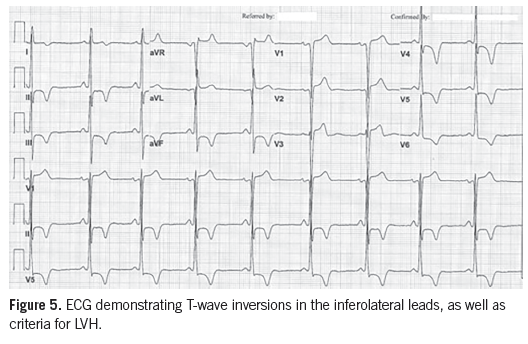
Case 2. A 54-year-old man with a history of hypertension, stage 2-3 chronic kidney disease, and an impaired fasting glucose was referred to the cardiology clinic after results of an ECG obtained during a preoperative evaluation were abnormal.
Diagnostic tests. The 12-lead ECG demonstrated changes consistent with LVH and repolarization abnormalities, including deep T-wave inversions in the lateral precordial leads (Figure 5). An echocardiogram showed moderate LVH with papillary muscle hypertrophy and apical hypertrophy (Figures 6A and 6B).
A year later, the patient presented with paroxysmal atrial fibrillation with rapid ventricular response, along with chest pain that resolved with rate control; cardiac marker test results were positive. Cardiac catheterization revealed nonobstructive coronary artery disease, and a spade-like configuration was noted on ventriculography (Figures 7A and 7B). Further assessment revealed a history of near-syncope, and a treadmill stress test showed symptomatic exercise-induced hypotension. The near-syncope and exertional hypotension were believed to be related to a midcavity obstruction and portended an increased risk for sudden cardiac death; therefore, an implantable defibrillator was placed.

Discussion. HCM is an autosomal dominant disease affecting the myocardium. This disease’s morphologic expression and natural progression can vary. Apical HCM, first reported in Japan in 1976 and also known as Yamaguchi syndrome, is an uncommon form of HCM in which the hypertrophied myocardium is localized to the apex of the LV.1
Both a pure apical form and distal-dominant form of this condition have been described. Patients with the distal-dominant form of aHCM more often are symptomatic and are more likely to experience cardiovascular events,2,3 although both the pure apical and distal dominant forms may have significant cardiovascular morbidity and mortality. These include atrial fibrillation and myocardial infarction, along with more uncommon complications such as apical infarction with apical aneurysm, ventricular arrhythmias, and sudden cardiac death. The majority of patients do not exhibit any decline in functional class, and approximately half remain asymptomatic on follow-up.4
ECG features of aHCM. The features of aHCM have been well-described in the literature in patients who had left cardiac catheterization for evaluation of either ischemic heart disease or cardiomyopathy.5 The ECG demonstrates giant negative T waves associated with high QRS voltage in the lateral precordial leads despite the absence of hypertension or significant coronary artery disease. A characteristic spade-like configuration of the LV has been observed in the right anterior oblique projection of the ventriculogram.3
A relationship between the depth of inverted T waves and the apex/midwall thickness ratio has been shown to suggest that the abnormal repolarization of the hypertrophied apical musculature causes these giant T-wave inversions.1 Approximately one-third of patients with aHCM experience adverse cardiovascular events such as myocardial infarction, atrial fibrillation, and CVA. Both of the patients described in this report later developed atrial fibrillation.
The differential diagnosis for the giant T-wave inversions on ECG that are characteristic of aHCM is broad. The causes of T-wave inversions may be benign or pathologic. In coronary artery disease, T-wave inversions may be present as a result of myocardial ischemia, a non-–ST segment myocardial infarction, and past myocardial infarction. T-wave changes related to ischemia usually are narrow and symmetric. When interpreting these ECG changes, the history and physical examination are paramount.
Prominent, deeply inverted, and wide T waves are more typical of noninfarction and nonischemic conditions such as CVA, pulmonary embolism with right ventricular strain, and LVH. Approximately 70% of patients with LVH will have ST-segment/T-wave changes that represent the altered repolarization of the ventricular myocardium. There is significant variability in the manifestation of this strain pattern on ECG; T waves may be minimally inverted, or greater than 5 mm in depth. Other features of LVH-related repolarization changes include depression of the J point, asymmetry of the T wave with rapid return to baseline, terminal positivity of the T wave, T-wave inversions in lead V6 greater than 3 mm, and T-wave inversions greater in lead V6 than in lead V4.6
The T-wave changes specifically associated with aHCM and localized LVH include deeply inverted T waves in the left precordial leads that are generally 1.2 mV or more. While T-wave inversions may be seen in ischemia, the T-wave changes associated with aHCM generally are deeply inverted and of obscure origin, without associated ischemic episode, arrhythmia, or CVA.1
Imaging features of aHCM. Echocardiography is a noninvasive imaging modality that may be helpful in identifying aHCM as well as in distinguishing the pure apical form of the condition from the distal-dominant form. Unfortunately, if the apex is not well-visualized, the diagnosis of aHCM may be missed. Kubo and colleagues2 used echocardiography in a cohort of 264 patients to further define the imaging characteristics of these 2 forms of aHCM. They found that 30% of the subjects were classified as having the apical phenotype,19% had the pure-apical form, and 11% had the distal-dominant form. LV systolic function was preserved in all patients with the apical phenotype. In patients with the distal-dominant form, the left atrial diameter was larger and the ratio of early and late ventricular filling velocity (E/Ea ratio) was higher than in patients with the pure apical phenotype. No patients showed LV outflow tract obstruction; however, midventricular obstruction or apical obliteration was frequently found in patients with the distal-dominant form.
Chen et al3 utilized 2-dimensional echocardiography to further classify aHCM. Diagnostic criteria for aHCM included LVH predominantly in the region of the LV apex, with a wall thickness greater than 15 mm and a ratio of apical to posterior wall thickness greater than 1.5. Distribution of the hypertrophied musculature further allowed for distinguishing between the pure apical form versus the distal-dominant form. The pure form was defined as hypertrophy limited to the apical portion of the LV below the papillary muscle; the distal-dominant form had simultaneous hypertrophy of other segments.
Paradoxic diastolic jet flow has been found to be an important marker of apical asynergy and an indication of an increased risk of adverse clinical events. The elevated diastolic apical pressure suggested by the flow may contribute to the development of an apical aneurysm.7
Cardiac catheterization with left ventriculography may be utilized to validate echocardiographic findings; it also is deemed the gold standard for diagnosing apical aneurysm and apical sequestration. Cardiac catheterization is not a routine examination in aHCM patients; however, it frequently is performed due to the similarity of its symptoms and ECG findings to those of coronary artery disease.
Yamaguchi and colleagues5 demonstrated that 30 patients with giant negative T waves uniformly showed an apical hypertrophic configuration at end diastole and dynamic symmetric contraction in systole. The silhouette of the LV is spade shaped, in contrast to the silhouette in HCM, which resembles a banana.5
Cardiac morphology may further be evaluated with cardiac magnetic resonance (CMR). This imaging modality may be used to measure LV dimensions, volumes, and ejection fraction. In patients with HCM, CMR can identify the pattern and extent of hypertrophy. CMR has been shown to be very useful in the evaluation of aHCM and other forms of HCM, especially when echocardiographic images are limited.8 CMR may be utilized to differentiate among the 3 types of aHCM—the true apical form, a type with additional asymmetric involvement of the ventricular wall segments, and a type with symmetric involvement of the ventricular wall segments. The spade shape of the ventricle is not present in the early stage of the disease but develops later.9
Kim and colleagues10 attempted to define the frequency and distribution of late gadolinium enhancement (LGE) on CMR and its prognostic implication in asymptomatic or minimally symptomatic aHCM patients. LGE often was observed in the thickened apex of the heart as well as the other LV segments, irrespective of the presence or absence of hypertrophy, and was not associated with an adverse prognosis in the asymptomatic or minimally symptomatic population.
Patients with symptomatic aHCM have been found to have hyper-enhancing apical segments on delayed-enhancement magnetic resonance imaging.11 A subendocardial hyper-enhancement pattern of the apical myocardium was observed and was associated with regional systolic dysfunction and significant ventricular arrhythmias in patients with symptomatic aHCM.11

Management of aHCM. As per American College of Cardiology Foundation/American Heart Association guidelines, patients with HCM and atrial fibrillation should be placed on oral anticoagulation just as other patients at high risk for thromboembolism in the setting of atrial fibrillation.12 The extent of hypertrophied myocardium has been found to play a role in the prediction of clinical or electromechanical characteristics such as the development of atrial fibrillation.13
These 2 cases demonstrate this uncommon clinical entity along with characteristic ECG, echocardiography, and ventriculography findings. No specific guidelines currently outline the management of patients with aHCM. In current practice, the management of aHCM patients is similar to that of patients with HCM, including stress testing to evaluate for symptoms related to dynamic midcavity obstruction. Furthermore, CMR may be utilized to identify the type and progression of the aHCM, although CMR was not obtained in the patients’ cases described here.
Improved and more commonly utilized imaging modalities will increase detection of this uncommon variant of HCM. Further research is needed to more clearly define the natural history of aHCM and to provide recommendations for its evaluation and management. For now, this condition should be considered when encountering a 12-lead ECG showing giant T-wave inversions, especially when acute ischemic conditions have been excluded or are unlikely.
Courtney R. Usry, DO, is a staff internal medicine physician at Blanchfield Army Community Hospital in Fort Campbell, KY, and a captain in the U.S. Army.
Sean P. Javaheri, DO, is an interventional cardiologist at Georgia Regents Heart and Cardiovascular Services in Augusta, GA, and has served as a physician in the U.S. Army.
The opinions expressed here are solely those of the individual authors and do not necessarily reflect those of the U.S. Government, the Department of Defense, or the U.S. Army.
REFERENCES:
Sakamoto T, Tei C, Murayama M, et al. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle: echocardiographic and ultrasono-cardiotomographic study. Jpn Heart J. 1976;17(5):611-629.
Kubo T, Kitaoka H, Okawa M, et al. Clinical profiles of hypertrophic cardiomyopathy with apical phenotype—comparison of pure-apical form and distal-dominant form. Circ J. 2009;73(12):2330-2336.
Chen C-C, Lei M-H, Hsu Y-C, Chung S-L, Sung Y-J. Apical hypertrophic cardiomyopathy: correlations between echocardiographic parameters, angiographic left ventricular morphology, and clinical outcomes. Clin Cardiol. 2011;34(4):233-238.
Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39(4):638-645.
Yamaguchi H, Ishimura T, Nishiyama S, et al. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol. 1979;44(3):401-412.
Hayden GE, Brady WJ, Perron AD, Somers MP, Mattu A. Electrocardiographic T-wave inversions: differential diagnosis in the chest pain patient. Am J Emerg Med. 2002;20(3):252-262.
Nakamura T, Matsubara K, Furukawa K, et al. Diastolic paradoxic jet flow in patients with hypertrophic cardiomyopathy: evidence of concealed apical asynergy with cavity obliteration. J Am Coll Cardiol. 1992;19(3):516-524.
Nagueh SF, Mahmarian JJ. Noninvasive cardiac imaging in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;48(12):2410-2422.
Higgins CB, de Roos A. Cardiovascular MRI and MRA. Philadelphia, PA: Lippincott Williams & Williams; 2003:107-108.
Kim K-H, Kim H-K, Hwang I-C, et al. Myocardial scarring on cardiovascular magnetic resonance in asymptomatic or minimally symptomatic patients with “pure” apical hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2012;14:52.
Amano Y, Takayama M, Fukushima Y, et al. Delayed-enhancement MRI of apical hypertrophic cardiomyopathy: assessment of the intramural distribution and comparison with clinical symptoms, ventricular arrhythmias, and cine MRI. Acta Radiol. 2011;52(6):613-618.
Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58(25):e212-e260.
Choi E-Y, Rim S-J, Ha J-W, et al. Phenotypic spectrum and clinical characteristics of apical hypertrophic cardiomyopathy: multicenter echo-Doppler study. Cardiology. 2008;110(1):53-61.


