Peer Reviewed
Noncommunicating (Obstructive) Hydrocephalus
Authors:
Justin Hahn, BS, OMS-IV
Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida
Kristina Rajic, BS, PA-S
Barry University, Miami Shores, Florida
Simone Phang-Lyn, MS, OMS-IV
Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida
Nada A. Saleh, MS-V
University of Jordan, Amman, Jordan
Syed A. A. Rizvi, PhD, MS, MBA
Hampton University School of Pharmacy, Hampton, Virginia
Zafar Qureshi, MD
UHI CommunityCare Clinic, Miami, Florida
Citation:
Hahn J, Rajic K, Phang-Lyn S, Saleh NA, Rizvi SAA, Qureshi Z. Noncommunicating (obstructive) hydrocephalus. Consultant. 2020;60(3):89-91. doi:10.25270/con.2020.03.00006
A 7-year-old girl with no relevant medical history presented to the emergency department (ED) for evaluation of loss of consciousness. The patient’s mother reported that the girl had a 1-day history of headache and multiple episodes of nonbilious, nonbloody vomiting.
History. According to the patient’s mother, the patient had had intermittent headaches over the past year, but she had not had severe headaches, headaches resistant to medical therapy, developmental delay, or behavioral changes. The mother reported that the girl had had a seizure lasting less than 10 minutes with no reported jerking. The mother claimed that there had been concerns about brain development in utero, but she said that follow-up magnetic resonance imaging (MRI) studies had shown no abnormalities. The family history was significant for sickle cell disease in a maternal aunt. Of note, the patient had not had regular visits to a pediatrician; due to inadequate regular follow-up visits, head circumference data for the child were not available until after age 6.
Physical examination. Vital signs in the ED were as follows: temperature, 36.4°C; heart rate, 90 beats/min; respiratory rate, 18 breaths/min; blood pressure, 128/70 mm Hg; oxygen saturation, 99% on room air; and weight, 30.3 kg. The patient was sleepy but easily arousable, had intact patellar reflexes, had no focal sensory or motor deficits, had a normal Babinski reflex, and had no clonus or signs of meningismus.
Diagnostic tests. Results of blood tests indicated normal sodium and potassium levels; normal prothrombin time (PT), international normalized ratio (INR), and activated partial thromboplastin time (aPTT); a normal fibrinogen level; and normal white blood cell (WBC) and red blood cell (RBC) counts.
Computed tomography of the brain without contrast showed dysmorphic-appearing ventricles with an appearance suggestive of lobar holoprosencephaly, evidence of hydrocephalus with dilation of the lateral ventricles, and a relatively normal-caliber third and fourth ventricle. Findings also showed no hemorrhage, mass effect, or evidence of impending herniation.
MRI of the brain with and without contrast showed obstructive hydrocephalus and cystic structure in the right lateral ventricle, causing mass effect of the third ventricle, tectum, midbrain, and cerebellum, with cerebellar tonsillar displacement. Findings also showed no enhancing mass within the brain parenchyma or leptomeninges following gadolinium administration.
The results of a subsequent lumbar puncture showed no WBCs and clear and colorless cerebrospinal fluid (CSF); the glucose level was mildly elevated, and the protein level was mildly decreased—both were nonsignificant findings. The results showed no signs of bacterial or viral meningitis. Due to her family history of sickle cell disease, hemoglobin electrophoresis was done, the results of which revealed that the hemoglobin A and A2 types were normal, with no signs of hemoglobinopathy (eg, sickle cell, thalassemia, etc).
A consulting ophthalmologist noted papilledema, most likely secondary to hydrocephalus, and retinal hemorrhage in the right eye, likely secondary to increased intracranial pressure.
She was taken to the operating room for cyst fenestration and extraventricular drain (EVD) placement. Postoperative fast MRI of the brain showed that the right lateral ventricle remained dilated with interstitial edema but had decreased in size compared with the results of the earlier study (Figure 1).
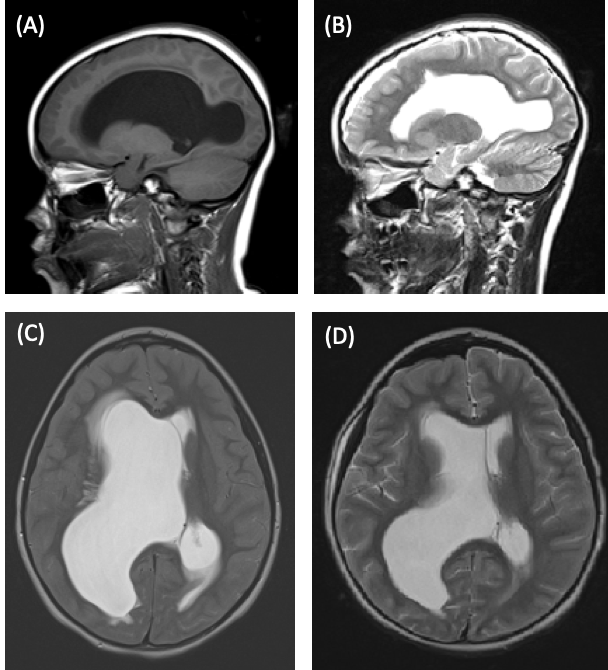
Figure 1. Preoperative sagittal (A) and transverse (C) MRI scans showing a dilated right lateral ventricle. Postoperative sagittal (B) and transverse (D) MRI scans showing a dilated but reduced in size right lateral ventricle.
Subsequent electroencephalography showed abnormal activity with no seizures. The patient’s neurologic activity improved clinically, progressing back to baseline, and dexamethasone and levetiracetam were initiated.
Differential diagnoses upon admission to the ED included an infectious etiology and the potential for meningitis, central nervous system tumor, trauma, and ingestion of a foreign substance. However, the patient’s vital signs, complete blood cell count (CBC) and differential findings, and serum electrolyte levels remained stable and within normal limits. Lumbar puncture results were unremarkable. An intracranial bleed or subarachnoid hemorrhage was ruled out based on results of the brain CT without contrast, which did not show process of an acute bleed or apparent hemorrhage. Hemoglobin electrophoresis and normal PT/INR and aPTT lowered the differential for coagulopathies. Intracranial hypertension and leukemia remained lower on the differential list due to the normal CBC and differential results and vital signs. Lumbar puncture results were unremarkable, and CSF cultures and Gram stain remained negative for evidence pathogens during the patient’s hospital stay.
Discussion. Hydrocephalus is the result of buildup of CSF in the ventricles. It is among the most common neurosurgical disorders. Hydrocephalus can be classified as either communicating (nonobstructive) or noncommunicating (obstructive).1,2 The etiology of obstructive hydrocephalus includes congenital or acquired causes. Congenital causes include neural tube defects, CNS malformations, and intrauterine infections. Acquired causes include hemorrhage, trauma, CNS tumors, cysts, or CNS infections. The common presenting symptoms are headache, blurred vision, nausea, irritability, decreased level of coordination, and, in infants, accelerated head growth and bulging fontanels.1,3
The diagnosis is based on the clinical symptoms (headache, vomiting, behavioral changes, seizure episodes) and findings from radiologic imaging. Management is based on the cause and correcting the hydrocephalus via CSF drainage and placement of ventriculoperitoneal (VP) shunt (if the cause of hydrocephalus is not eliminated).4-6
For our patient, medical management (dexamethasone and levetiracetam) was optimized to reduce periventricular edema and provide seizure prophylaxis. Her hospital course included multiple failed clamp trials of the EVD, and a VP shunt was placed. She has been doing well clinically and has had minimal complications since surgical intervention (Figure 2). She is scheduled to follow up with ophthalmology and neurology specialists.

Figure 2. VP shunt placed on the right side of the patient’s head.
- Bhatia MS, Srivastava S, Saha R, Gautam P. Noncommunicating hydrocephalus presenting with pseudoseizures: a case report. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15l01827.
- Pople IK. Hydrocephalus and shunts: what the neurologist should know. J Neurol Neurosurg Psychiatry. 2002;73(suppl 1):i17-i2
- Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387(10020):788-799.
- Filis AK, Aghayev K, Vrionis FD. Cerebrospinal fluid and hydrocephalus: physiology, diagnosis, and treatment. Cancer Control. 2017; 4(1):6-8.
- Vinchon M, Rekate H, Kulkarni AV. Pediatric hydrocephalus outcomes: a review. Fluids Barriers CNS. 2012;9(1):18.
- AbdelRazek MA, Venna N. Ventriculoperitoneal-shunt placement for normal-pressure hydrocephalus. N Engl J Med. 2017;377(26):e35.


