Peer Reviewed
Adrenal Incidentaloma: A Review for Primary Care
AUTHOR:
Jeffrey T. Budd, MD
AFFILIATION:
Department of Medicine, Division of General Internal Medicine, University of Florida College of Medicine, Gainesville, Florida
CITATION:
Budd JT. Adrenal incidentaloma: a review for primary care. Consultant. 2022;62(3):e1-e5. doi:10.25270/con.2021.07.00005
Received February 14, 2021. Accepted March 26, 2021. Published online July 16, 2021.
DISCLOSURES:
The author reports no relevant financial relationships.
CORRESPONDENCE:
Jeffrey Thomas Budd, MD, University of Florida College of Medicine, 1329 SW 16th Street, Suite 5140, Gainesville, FL 32610 (buddjt@medicine.ufl.edu)
Abstract
Incidental adrenal tumors are common but are most often asymptomatic. The challenge for primary care providers is to determine the hormone secretion status and malignancy potential. This article outlines the causes of incidental adrenal tumors, how to screen for and treat each cause, and the follow-up management of incidental adrenal tumors.
Introduction
Adrenal incidentalomas are nodules measuring 1 cm or greater that are discovered by imaging ordered for reasons other than suspected adrenal disease.1 These are common findings with an estimated prevalence of 1.05% to 8.70% of adults.2 Advances in imaging technology and the more widespread use of imaging have resulted in an increased detection of adrenal incidentalomas.2-4 The clinical challenge is to differentiate harmless nodules from malignancy and hormone-secreting masses.
Most adrenal incidentalomas are benign adrenal adenomas (80%) and are nonfunctional (75%).5 Primary adrenal cancer is rare, at 1.9% of adrenal incidentaloma diagnoses.6 The most common endocrine disease associated with secreting adrenal incidentalomas is subclinical Cushing syndrome, and a less likely disease is silent pheochromocytoma or hyperaldosteronism. Unilateral adrenal nodules appear to be equally prevalent between adrenal glands on both the left and right sides, and 10% to 15% of nodules are bilateral.7
Adrenal incidentalomas are rarely found in patients younger than age 30 years and appear to be most prevalent in patients aged 50 to 80 years.8 The average age at diagnosis is 57 years.8 Although asymptomatic adrenal masses are found with equal gender distribution at autopsy, slightly more women have adrenal incidentalomas discovered by imaging.7 This gender difference may be due to more frequent abdominal diagnostic imaging in women.7 Adrenal masses may be more prevalent in patients with obesity, hypertension, or type 2 diabetes.5 An approach to screening for adrenal incidentalomas is shown in the Figure.

Figure. Approach to Screening for Adrenal Incidentaloma
Note: DST, dexamethasone suppression test
Excess Hormone Production
For patients with recently detected adrenal incidentalomas, a detailed evaluation should focus on the symptoms and signs of overt adrenal hormone excess (Table).9 Hypersecretion may be more prevalent in bilateral adrenal incidentalomas or adrenal masses measuring 4 cm or larger.7 Because hypercortisolism or catecholamine overproduction can be clinically silent but associated with adverse outcomes, screening is necessary for both hypercortisolism and catecholamine overproduction.5,10
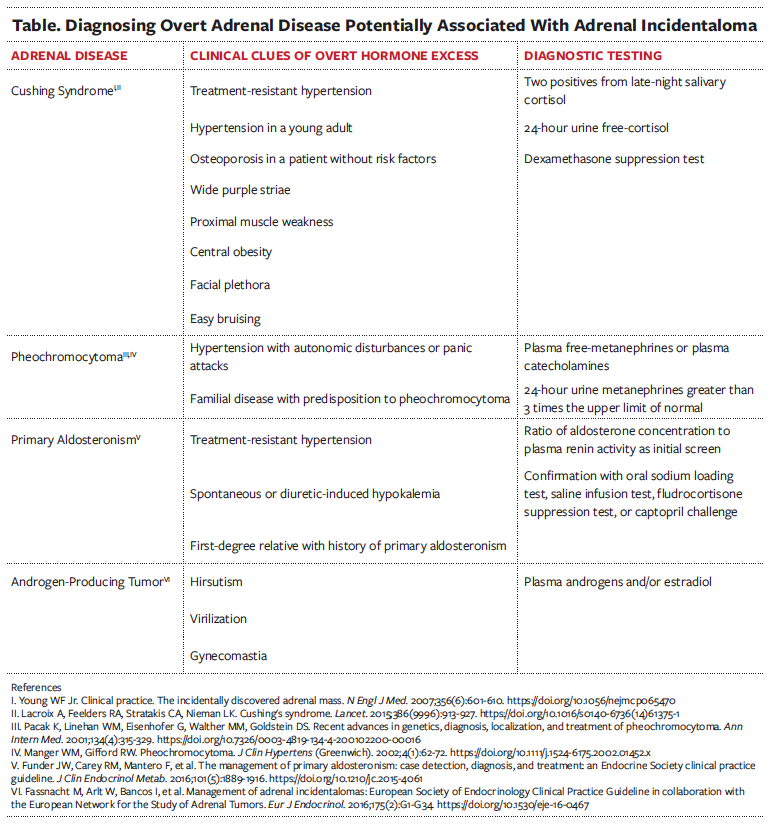
Subclinical Cushing Syndrome
Subclinical hypercortisolism is present in approximately 6.4% of adrenal incidentalomas and is the most frequent hormone abnormality.6,7,11 Development of overt Cushing syndrome is not the primary concern, because very few (< 1%) subclinical cases progress.9 Instead, the clinical concern for subclinical Cushing syndrome is the associated increased risks of cardiovascular disease and osteoporosis.10,12,13 Subclinical Cushing syndrome is associated with insulin resistance, type 2 diabetes, hypertension, and dyslipidemia.10,12
Subclinical Cushing syndrome can be excluded with laboratory test results showing a plasma cortisol level of less than or equal to 1.8 μg/dL from a 1-mg overnight dexamethasone suppression test.9 For cortisol levels greater than 1.8 μg/dL, the diagnosis of subclinical Cushing syndrome needs to be confirmed with a repeat dexamethasone suppression test within 3 to 12 months and additional tests for levels of plasma adrenocorticotropic hormone (ACTH), late-night salivary cortisol, or 24-hour urine free-cortisol.9 For patients who qualify for adrenalectomy, a plasma ACTH level is needed to confirm ACTH independency.9
Management options for subclinical Cushing syndrome are either medical control of comorbidities or adrenalectomy.14 Because there is a lack of high-quality data from studies, as well as no consensus in guidelines to select which patients should undergo a surgical procedure, the decision for adrenalectomy needs to be individualized and made with a multidisciplinary approach with an endocrinologist and endocrine surgeon.14 Because adrenal insufficiency is possible after adrenalectomy for patients with subclinical Cushing syndrome, perioperative stress-dose glucocorticoid replacement is required.9
Silent Pheochromocytoma
From autopsy data, more than 75% of all of pheochromocytomas are silent.7 These unrecognized or clinically silent pheochromocytomas are hazardous and may present suddenly and fatally as a myocardial infarction, a stroke, or perioperative death during unrelated surgical procedures.15 Pheochromocytomas associated with adrenal incidentalomas have been diagnosed more frequently in the past few decades and represent approximately 5% of all adrenal incidentalomas.10,16
Clinical guidelines recommend the initial hormonal function test should include measurements of plasma-free metanephrines or urinary-fractionated metanephrines.17 Normal findings from these tests will exclude a diagnosis of pheochromocytoma, but elevated findings, especially borderline elevations, do not conclusively diagnose pheochromocytoma.17 Because only about 75% of patients with increased plasma-free metanephrines or urinary-fractionated metanephrines have confirmed pheochromocytomas, consulting an endocrinologist can assist with interpreting the results and determine whether further testing is necessary.17 When a unilateral pheochromocytoma is confirmed, treatment is adrenalectomy.9
Primary Aldosteronism
Evaluation for primary aldosteronism is not routine for all adrenal incidentalomas. Instead, screening for hyperaldosteronism is recommended for patients with comorbid hypertension or unexplained hypokalemia. Testing usually includes measuring the ratio of plasma aldosterone (ng/dL) to renin activity (pg/mL/h).18 Confirmatory testing with either a saline infusion test, fludrocortisone suppression test, oral sodium loading test, or captopril challenge is indicated for patients with a ratio greater than 30 and an aldosterone concentration greater than 10 ng/dL, unless the aldosterone level is greater than 20 ng/dL, renin activity is below detectible limits, or there is spontaneous hypokalemia.15,18
Malignancy
Adrenocortical carcinomas account for 8% to 11% of adrenal incidentalomas, and metastases account for 5% to 7% of adrenal incidentalomas.19 Adrenocortical carcinomas are very rare, with an incidence of 0.72 per million cases per year and represents 0.2% of cancer deaths in the United States.20 The median age at diagnosis of adrenocortical carcinoma is 50 to 60 years, and women appear to be twice as likely to have adrenocortical carcinomas than men.20 No familial correlation, genetic predisposition, or risk factors have been identified.20
Metastases to the adrenal glands are common for several cancers, especially for melanoma, lung, colorectal, esophageal, gastric, and breast cancers.21 In patients with a known malignancy, approximately 32% to 73% of adrenal incidentalomas are metastases that are asymptomatic without symptoms or signs of adrenal insufficiency.21
Tumor size and Hounsfield unit value on noncontrast computed tomography (CT) scan are the first-line diagnostic approaches to evaluate an adrenal incidentaloma for malignancy.19 The Hounsfield scale is a quantitative representation of radiodensity where water and fat have lower values than higher-density tissues or bone. Scores of 10 Hounsfield units or less are consistent with lipid-rich, benign adrenal adenomas and are unlikely to be malignant.9,22,23 Tumor size also corresponds with the likelihood of malignancy, with sizes greater than 4 cm representing a 24% increased risk.10 Adrenal incidentalomas measuring 4 cm, 6 cm, or 8 cm have malignancy risks of 10%, 19%, and 47%, respectively.19 A homogenous adrenal incidentaloma with a density of 10 Hounsfield units or less and size less than 4 cm is most likely benign and requires no further work-up.19
Adrenal incidentalomas with radiodensities greater than 10 Hounsfield units can be further evaluated by CT scan with contrast-enhanced washout, because benign adrenal adenomas have a rapid loss of CT contrast compared with other adrenal nodules.19 Images are taken before contrast, then at 1 min and 10 or 15 min after contrast injection to calculate relative and absolute washout values. An absolute washout of greater than 60% combined with a relative washout greater than 40% are characteristics of an adenoma and require no further imaging.19,24
For adrenal incidentalomas with a Hounsfield unit value greater than 10, size greater than 4 cm, prolonged CT contrast-enhanced washout, or indeterminate CT characteristics, consulting an endocrinologist and/or surgeon can help determine whether additional magnetic resonance imaging or positron emission tomography (PET)/CT scanning is needed to further characterize the tumor, reimage in 6 to 12 months, or resect the tumor.19,25 PET/CT scanning may be especially useful for evaluating masses in patients with a history of cancer and indeterminate characteristics seen on noncontrast CT scan.19 Biopsy by fine-needle aspiration is not typically recommended for assessing an adrenal incidentaloma unless a patient has a history of extra-adrenal malignancy, no evidence of pheochromocytoma, or inconclusive characteristics on imaging scans, and management of the adrenal incidentaloma would be influenced by histology.19,21
Follow-Up Management
Development of malignancy or hormonal hypersecretion after a negative initial evaluation is rare. About 1% to 2% of nonfunctional adrenal masses later convert to hypersecretion upon follow-up, and evolution to malignancy may be as low as 0.2%.6,19,26 Moreover, false-positive findings upon follow-up may outnumber true positives and lead to unnecessary interventions and stress for the patient.6 Recommendations vary for management of adrenal incidentalomas after negative initial assessment. One option is no hormone follow-up if the work-up is negative unless future clinical evidence of hormonal excess arises, and no imaging follow-up is needed if benign characteristics are present on imaging scans.6,9 Another option is to monitor the patient with annual hormone screening for 4 years and noncontrast CT scanning at 6 months alone or at 6, 12, and 24 months.10,25

Conclusion
Incidental adrenal tumors are common and, most often, are benign. The challenge for primary care providers is to determine the hormone secretion status and malignancy potential. In asymptomatic adrenal masses, subclinical Cushing syndrome is the most common hormone dysfunction and is concerning because of the associated increased risks of cardiovascular disease and osteoporosis. Pheochromocytomas may be surprisingly clinically silent but have a high morbidity and mortality risk if missed. Testing for primary aldosteronism or androgen-producing tumors is reserved for patients with other findings that suggest them, such as comorbid hypertension, spontaneous hypokalemia, hirsutism, or virilization.
Primary adrenal cancer—adrenocortical carcinoma—is rare, but the adrenal glands are a prevalent location for metastases from other malignancies. Noncontrast CT scanning is the first-line diagnostic tool to evaluate an adrenal incidentaloma. A mass measuring less than 4 cm with a radiodensity at or below 10 Hounsfield units is most likely benign. Management of masses larger in size or with a greater radiodensity than 10 Hounsfield units requires an individualized approach with an endocrinologist and/or surgeon. Guidelines vary for further follow-up of adrenal nodules initially found to be benign and hormonally nonfunctional from clinical monitoring to serial hormone testing and imaging.
References
1. Kebebew E. Adrenal incidentaloma. N Engl J Med. 2021;384(16):1542-1551. https://doi.org/10.1056/nejmcp2031112
2. Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298-302. https://doi.org/10.1007/bf03344099
3. Muscogiuri G, De Martino MC, Negri M, et al. Adrenal mass: insight into pathogenesis and a common link with insulin resistance. Endocrinology. 2017;158(6):1527-1532. https://doi.org/10.1210/en.2016-1804
4. Jason DS, Oltmann SC. Evaluation of an adrenal incidentaloma. Surg Clin North Am. 2019;99(4):721-729. https://doi.org/10.1016/j.suc.2019.04.009
5. Terzolo M, Stigliano A, Chiodini I, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164(6):851-870. https://doi.org/10.1530/eje-10-1147
6. Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol. 2009;161(4):513-527. https://doi.org/10.1530/eje-09-0234
7. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149(4):273-285. https://doi.org/10.1530/eje.0.1490273
8. Sherlock M, Scarsbrook A, Abbas A, et al. Adrenal incidentaloma. Endocr Rev. 2020;41(6):775-820. https://doi.org/10.1210/endrev/bnaa008
9. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1-G34. https://doi.org/10.1530/eje-16-0467
10. Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601-610. https://doi.org/10.1056/nejmcp065470
11. Young WF Jr. Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am. 2000;29(1):159-x. https://doi.org/10.1016/s0889-8529(05)70122-5
12. Tauchmanovà L, Rossi R, Biondi B, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87(11):4872-4878. https://doi.org/10.1210/jc.2001-011766
13. Chiodini I, Morelli V, Masserini B, et al. Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J Clin Endocrinol Metab. 2009;94(9):3207-3214. https://doi.org/10.1210/jc.2009-0468
14. Hsieh LB, Mackinney E, Wang TS. When to intervene for subclinical Cushing's syndrome. Surg Clin North Am. 2019;99(4):747-758. https://doi.org/10.1016/j.suc.2019.04.011
15. Farrugia FA, Martikos G, Tzanetis P, et al. Pheochromocytoma, diagnosis and treatment: Review of the literature. Endocr Regul. 2017;51(3):168-181. https://doi.org/10.1515/enr-2017-0018
16. Clifton-Bligh R. Diagnosis of silent pheochromocytoma and paraganglioma. Expert Rev Endocrinol Metab. 2013;8(1):47-57. https://doi.org/10.1586/eem.12.76
17. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
18. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426-e483. https://doi.org/10.1161/cir.0000000000000597
19. Corssmit EPM, Dekkers OM. Screening in adrenal tumors. Curr Opin Oncol. 2019;31(3):243-246. https://doi.org/10.1097/cco.0000000000000528
20. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282-326. https://doi.org/10.1210/er.2013-1029
21. Spartalis E, Drikos I, Ioannidis A, et al. Metastatic carcinomas of the adrenal glands: from diagnosis to treatment. Anticancer Res. 2019;39(6):2699-2710. https://doi.org/10.21873/anticanres.13395
22. Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass ("incidentaloma"). Ann Intern Med. 2003;138(5):424-429. https://doi.org/10.7326/0003-4819-138-5-200303040-00013
23. Hamrahian AH, Ioachimescu AG, Remer EM, et al. Clinical utility of noncontrast computed tomography attenuation value (hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J Clin Endocrinol Metab. 2005;90(2):871-877. https://doi.org/10.1210/jc.2004-1627
24. Bhargava P, Sangster G, Haque K, Garrett J, Donato M, D'Agostino H. A multimodality review of adrenal tumors. Curr Probl Diagn Radiol. 2019;48(6):605-615. https://doi.org/10.1067/j.cpradiol.2018.10.002
25. NIH state-of-the-science statement on management of the clinically inapparent adrenal mass ("incidentaloma"). NIH Consens State Sci Statements. 2002;19(2):1-25. https://consensus.nih.gov/2002/2002AdrenalIncidentalomasos021Program.pdf
26. Bülow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma - follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154(3):419-423. https://doi.org/10.1530/eje.1.02110


