Peer Reviewed
Adult Obesity Management for Primary Care
AFFILIATION:
Department of Medicine, Division of General Internal Medicine, University of Florida College of Medicine, Gainesville, Florida
CITATION:
Budd JT. Adult obesity management for primary care. Consultant. 2022;62(12):e1. doi:10.25270/con.2022.07.000021
Received February 4, 2022. Accepted February 18, 2022. Published online July 29, 2022.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Jeffrey Thomas Budd, MD, University of Florida College of Medicine, 1329 SW 16th Street, Suite 5140, Gainesville, FL 32610 (buddjt@medicine.ufl.edu)
Abstract
Along with the global prevalence of obesity, the associated health and economic impacts are rapidly multiplying. The challenge to primary care is to identify and treat obesity as a chronic disease. Patients should receive specific advice about diet, lifestyle, and physical activity rather than a vague or terse recommendation to simply eat less and exercise. Regular monitoring and follow-up visits are critical, as with any chronic disease. When nonpharmacologic methods do not lead to weight loss, select patients should be offered supplementary pharmacotherapy. Bariatric surgery is a safe and effective treatment for some patients with persistent severe obesity or obesity with complications despite nonpharmacologic efforts and medications.
Introduction
The American Medical Association formally recognized obesity as a chronic disease in 20131 and, in doing so, also acknowledged that it has become an alarming public health threat.2 Overweight, obesity, and severe obesity are defined by a body mass index (BMI) of 25 to 30 kg/m2, 30 kg/m2 or more, and 40 kg/m2 or more, respectively.3 Obesity has an accelerating prevalence globally, with corresponding increases in health and economic burdens.2 Over the past 4 decades, obesity has almost tripled worldwide, and now most of the population lives in countries where mortality from obesity exceeds mortality from malnutrition.4 In 2016, 39% of adults worldwide had weights in the overweight range, and 13% had obesity.4
In the United States, 42.4% of adults have obesity and 9.2% have severe obesity.5 The prevalence of obesity in the United States is highest among Black adults (49.6%) and lowest among Asian adults (17.4%). In 2 decades, the prevalence of obesity in the United States has increased by 39% (from 30.5% to 42.4%) and the prevalence of severe obesity has increased by more than 96% (from 4.7% to 9.2%).5
Economic and Health Burdens
Obesity carries a higher risk of all-cause mortality, and this risk increases with rising BMI.6 The mortality risk is greater in men than in women.6 Most organ systems can be detrimentally affected7; consequently, obesity has numerous associated complications. Some of the more serious comorbidities include type 2 diabetes, hypertension, cardiovascular disease, cerebrovascular disease, obstructive sleep apnea, multiple cancers, nonalcoholic fatty liver disease, hyperlipidemia, polycystic ovary, urogenital disease, and gallbladder disease.2
Health care costs for patients with obesity are 36% greater overall, or approximately $600 more on average per year for Medicare patients compared with Medicare patients without obesity.8 The national annual cost for medical care of obesity and its comorbidities may be as high as $209.7 billion in the United States.9 The global yearly economic burden of obesity is an estimated $2.0 trillion, which is similar to the economic impacts of smoking ($2.1 trillion) or armed violence, war, and terrorism combined ($2.1 trillion).10
Screening for and Identification of Obesity
Patients should be screened at least annually for obesity with measurement of BMI.11,12 Nevertheless, BMI can be a misleading marker of body adiposity and its associated health risk when muscle mass is well outside of the norm. In sarcopenic obesity, which is more common in patients with advanced age, low muscle mass leads to a higher percentage of body adiposity and higher health risk than expected for the calculated BMI.7 Similarly, patients with high muscle mass have a lower percentage of body fat and lower health risk than expected for their BMI.7
BMI calculation alone also misses the contribution of adipose tissue distribution to health risk. Because abdominal obesity in particular carries a higher risk of cardiovascular disease, diabetes, and fatty liver disease,13 measuring waist circumference with BMI may be helpful.11,14 The current American Association of Clinical Endocrinology and American College of Endocrinology (AACE/ACE) guidelines11 recommend monitoring waist circumference for all patients with a BMI of less than 35 kg/m2. AACE/ACE guidelines identify patients at risk for abdominal adiposity as those with a waist circumference of 85 cm or more for Asian men and 102 cm or more for non-Asian men, and 74 cm or more for Asian women and 88 cm or more for non-Asian women.11
Nonpharmacologic Treatment
Despite obesity being well recognized as a chronic disease with escalating prevalence and worrisome health consequences, there remains substantial clinical inertia to address and manage it.15 Primary care providers and other health care practitioners may have more effective roles in curbing the obesity pandemic by making obesity a formal diagnosis, giving priority to weight loss and treatment discussions during a visit, understanding treatment options and their effectiveness, and providing follow-up visits to monitor obesity.15 Clinic visit time constraints and perceived lower priority status may partially explain why health care practitioners do not have weight-related conversations or do not schedule follow-up appointments to readdress the issue when such conversations do take place.15
Obesity is a chronic disease with varying contributions from genetics, environmental factors, metabolism, and behavior; the disease is much more complex than the mistaken view that it is simply a result of poor diet choices, lack of self-control, and laziness.2 Most patients understand that weight loss improves health and well-being. Rather than simply recommending weight loss, a health care practitioner should consider discussing specific treatment options and goals, similar to any other chronic disease.7 Patients often need guidance from their physicians in setting realistic weight-loss goals.7 Targeting loss to ideal body weight or a specific BMI may not be necessary because even a modest 5% to 10% loss leads to benefits in blood pressure, cholesterol, blood sugar, and quality of life.16,17
A single, uniform treatment path for all patients with obesity does not exist. Instead, and perhaps most important, each treatment plan needs to be individualized with recognition of social and cultural factors as well as patient preferences and integration with other aspects of a patient’s health care.18 An approach to the management of obesity is summarized in the Figure.
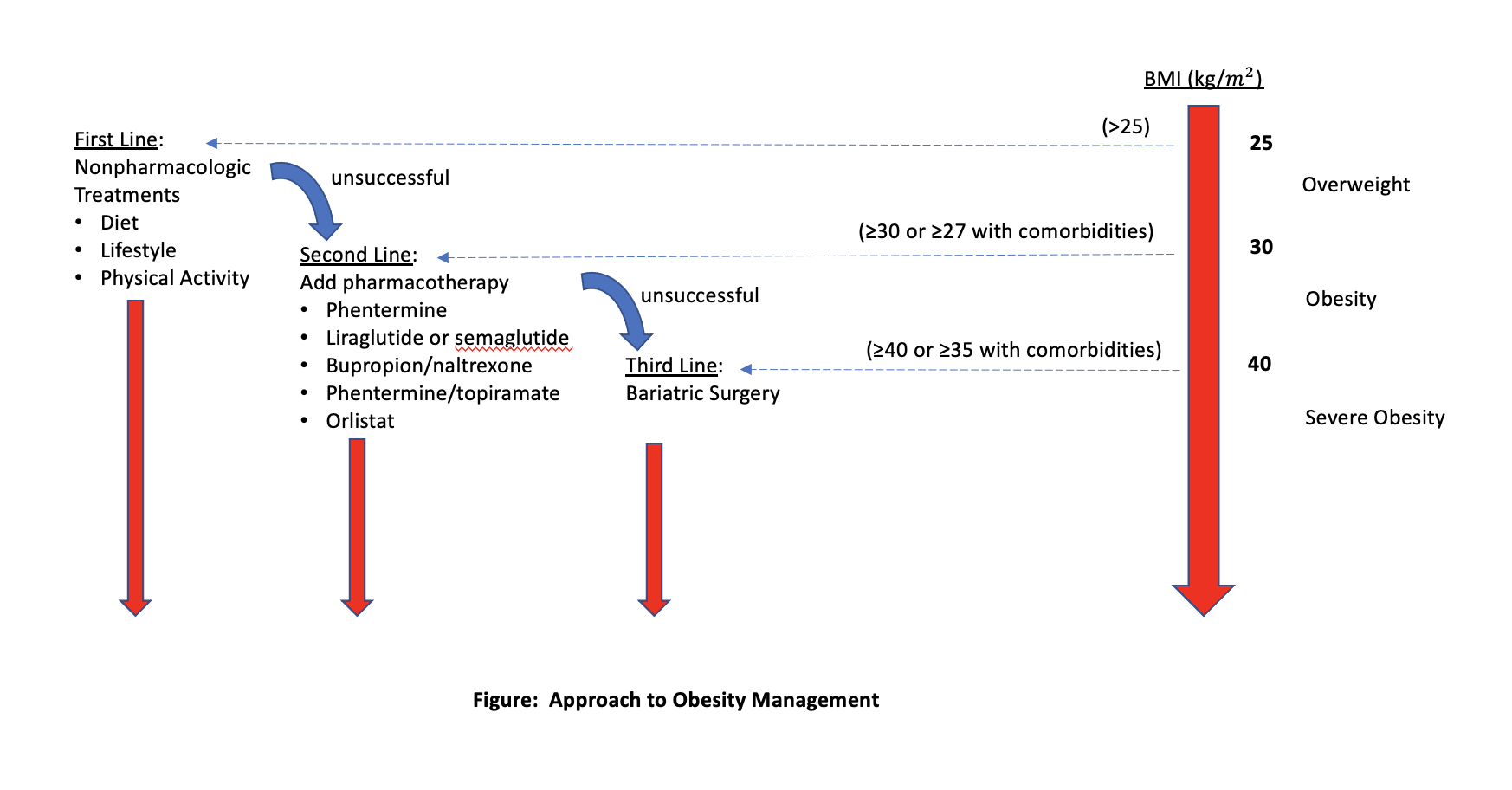
Figure. Approach to obesity management. Credit: Jeffrey T. Budd, MD
Behavior and Comprehensive Lifestyle Modification
The common but oversimplified advice to “just eat less and exercise more” is not typically helpful.17 In fact, initial weight loss progressively decelerates in most attempts and weight rebound is common.16 Addressing the foundational behaviors and lifestyles is important for the improved health of and sustained weight loss in patients with obesity.12,16,17,19-21 Success at reaching a goal of 5% to 10% weight loss is best when a structured behavioral plan, a reduced-calorie diet, and increased physical activity are managed with a trained interventionalist in group or individual meetings for 6 months with an initial weekly frequency.12 Some commercial programs to address lifestyle intervention are efficacious.22 Amplifying lifestyle intervention efforts may be helpful for patients who do not lose 2.5% of weight in the first month of treatment.11 The Centers for Disease Control and Prevention provides useful tips for lifestyle and behavioral changes to develop healthy eating and healthy weight.23
Diet
Although many diet types are available and several may be very popular at any given time, most diets are equally successful as long a negative energy balance is created and sustained adherence is achieved.12,16,17,24 A reasonable approach is to not only emphasize calorie reduction but also a well-balanced diet for both long-term healthy eating habits and a healthy weight. Most patients can achieve a calorie deficit and weight loss by targeting a daily calorie count of 1200 to 1500 for women and 1500 to 1800 for men.25 The MyPlate Plan from the US Department of Agriculture can be useful developing an initial, individualized calorie target based on age, sex, weight, height, and level of physical activity.26 This resource is also helpful for selecting meals to include healthy options from all food groups and keep within a calorie allowance. The Centers for Disease Control and Prevention also provides suggestions for meal planning, cutting calories, and healthy recipes.23 Some key diet points to highlight for patients are (1) that any healthy diet plan can be customized to reflect personal preferences, cultural traditions, and budgets; (2) that core elements of a healthy and balanced diet include vegetables, fruits, dairy, grains (half or more from whole grain), protein, and oils (vegetable oil and oils naturally found in seafood and nuts); and (3) to limit added sugars to less than 10% of calories, saturated fats to less than 10% of calories, sodium to 2300 mg or less daily, and alcohol to 2 drinks daily or less for men and 1 drink for women.27
Most patients will benefit from continued encouragement and reinforcement of long-term goals because, with any diet, weight loss levels off after 3 to 6 months, and most patients regain some weight over time, even with strict observance of a diet plan.7 Long-term adaptations of appetite and metabolism are likely responsible.7 The reduction in metabolism can be out of proportion to expected for body weight alone, and intensified hunger cravings tend to persist rather than wane.7
Physical Activity
Aerobic training can lead to modest weight loss, more so than resistance training.28 However, the health benefits of physical activity extend well beyond the potential for weight loss with reduced all-cause mortality and lower risks of heart disease, stroke, hypertension, type 2 diabetes, dementia, hyperlipidemia, and several cancers (bladder, breast, colon, endometrial, esophagus, renal cell, lung, and gastric).29 So, combining a well-balanced diet and physical activity is an ideal goal for all patients and promotes an overall healthy lifestyle.
The specific amount of activity needed to lose or maintain body weight may vary considerably by patient29 and the coexisting diet being followed. The US Department of Health and Human Services recommends at least 150 minutes per week of moderate-intensity exercise or 75 minutes of vigorous-intensity exercise for all persons, but some patients attempting to lose or maintain weight may require more. Despite these recommendations, a key point to emphasize to patients is that any amount of activity has health benefits over no activity29 even if ideal weight loss does not occur.
Going beyond the minimum recommendation of 150 minutes weekly of moderate-intensity exercise—up to 300 minutes weekly at moderate intensity and adding moderate muscle-strengthening exercises with all major muscle groups for 2 days weekly—can further boost health benefits.29 Examples of moderate-intensity aerobic activities are gardening, walking 2.5 m/hr, and biking slower than 10 m/hr; examples of more vigorous activities are biking 10 m/hr or more, jumping rope, swimming laps, and running.30 Muscle strengthening should ideally involve the legs, hips, back, abdomen, chest, and arms.23 Examples of moderate muscle-strengthening exercises are 8 to 12 repetitions (to exhaustion) of lifting weights, resistance bands, push-ups, or sit-ups.23 Others may include yoga and heavy gardening with shoveling.23
Pharmacotherapy
Medications may be indicated when diet and exercise have been unsuccessful and the BMI is 30 kg/m2 or more or 27 kg/m2 or more with obesity-related complications.3,7,17,20 Despite evidence of benefit from pharmacotherapy, physicians tend to undervalue prescription medication compared to diet and exercise,31 and only an estimated 2% of patients meeting criteria for pharmacotherapy receive treatment.32 In fact, 58% of primary care providers report a negative impression of medication for obesity, 60% do not prescribe medications for short-term weight loss (< 3 months), and 76% do not prescribe for long-term weight loss.33
Current guidelines are lacking in specific duration of pharmacotherapy. Because short-term treatment of 6 months or less may not produce meaningful, long-standing health benefits and weight regain is common when medications are stopped, pharmacotherapy for obesity is likely a long-term option.7,11 Pharmacotherapy is also an adjunct to lifestyle modification instead of a replacement, because combination therapy has been shown to lead to greater weight loss than pharmacotherapy alone.7,11
Orlistat, liraglutide, semaglutide, phentermine-topiramate, and naltrexone-bupropion are currently approved by the US Food and Drug Administration (FDA) for the chronic management of obesity. Phentermine is FDA-approved for short-term use up to 3 months. Each medication option alone results in significant weight loss over placebo, but the amount of weight loss varies.16 After a year of treatment, average weight loss over placebo is 8.8 kg with phentermine-topiramate, 5.3 kg with liraglutide, 5 kg with naltrexone-bupropion, and 2.6 kg with orlistat.34 At 17 months, an average 10.6 kg of weight loss may be achieved over placebo with semaglutide as an adjunct to intensive behavioral therapy.35 Average weight loss with phentermine monotherapy is 3 kg at 3 months relative to placebo.36
After 3 to 4 months of treatment, a new plan may be needed if weight loss is less than 4% to 5% in patients without diabetes or less than 3% of total body weight in patients with diabetes.37 Specific drug safety issues, insurance coverage, cost, and patient preference are factors in selecting a medication.16 Several of the newer drugs are costly and a potential source of socioeconomic disparity.38
Phentermine and Topiramate
Phentermine has the lowest cost and is the most widely prescribed weight-loss medication in the United States.7 Because it is a sympathomimetic, phentermine is typically not recommended for use in patients with poorly controlled hypertension or heart disease.20 Although little long-term evidence exists for the safety and efficacy of phentermine monotherapy, use beyond 3 months may be an option when significant weight loss (≥ 5%) is maintained, blood pressure remains controlled, no contraindications develop, and the patient understands that the medication is not FDA-approved for long-term use.7,20,39,40
The combination of low-dose phentermine and topiramate boosts the weight loss from either agent vs monotherapy alone, with fewer adverse effects.7,38,39 Several caveats to the use of this combination medication are contraindications with heart disease or uncontrolled hypertension from the phentermine component and the risks of teratogenicity, cognitive or psychiatric adverse effects, and metabolic acidosis from the topiramate component.7,39 As a result of the teratogenic potential, phentermine-topiramate has an FDA-required Risk Evaluation and Mitigation Strategy for patients, prescribers, and pharmacies.41
Naltrexone and Bupropion
The combination of naltrexone and bupropion reduces food craving through its effects on the hypothalamus in reducing appetite and on the mesolimbic dopamine circuit in suppressing the reward system.42 Treatment should be started at a low dose of naltrexone, 8 mg, and bupropion, 90 mg, daily and increased gradually to minimize nausea and other adverse effects.43 Concurrent opiate use or withdrawal, seizure disorder, renal failure, and eating disorders are some contraindications to the use of naltrexone-bupropion.
Orlistat
Orlistat impairs absorption of ingested fats by inhibiting digestive enzymes.39 Unfortunately, the relatively high rate of adverse effects (ie, fecal urgency, fecal incontinence, oily spotting)44 limit its popularity and use.38,39 Because orlistat may increase urine oxalate concentrations, caution should be taken for use in patients with a history of oxalate stones.40 Low-dose orlistat is sold over-the-counter.
Semaglutide and Liraglutide
Semaglutide and liraglutide are injectable glucagon-like peptide-1 (GLP-1) receptor agonists that inhibit weight loss by reducing appetite and slowing gastric emptying.7 Although initial post-marketing reports suggested an increased risk of pancreatitis with GLP-1 agonists, more recent data do not show a significant association with either pancreatitis or pancreatic cancer.39,45 Their use is contraindicated for patients with family histories of multiple endocrine neoplasia type 2 or personal history of medullary thyroid cancer.7
Hydrogel
A superabsorbent hydrogel from modified cellulose and citric acid is the most recent FDA-approved treatment for obesity, and interestingly, it is classified as a medical device instead of a drug because its mechanism of action is mechanical.46 As a hydrogel, it expands with water in the stomach to produce a sensation of satiety and fullness and is later broken down in the colon to release the water and return to a cellulosic matter to be expelled in feces.46 It is not absorbed systemically and has no apparent increased safety risks.47 At 24 weeks, average weight loss is modest at 2.4% vs placebo.47
Surgical Options
Bariatric surgery has superior outcomes to lifestyle, diet, and pharmacotherapy and is the most successful method for initial weight loss and maintained weight loss.16 Gastric bypass, vertical-banded gastropathy, and banding can produce weight losses of up to 32%, 25%, and 20%, respectively, at 1 to 2 years and sustained weight losses of 25%, 16%, and 14%, respectively, at 10 years.48 Bariatric surgery may be indicated for patients with a BMI of 40 kg/m2 or more or 35 kg/m2 or more with obesity-related complications.7,11,12,17 Bariatric surgery is a safe option17 with an estimated low mortality rate of 0.04% to 0.30% and a complication rate of 1% to 4%.49 Monitoring for nutritional deficiencies, especially for iron and vitamin D, and the avoidance of nonsteroidal anti-inflammatory drugs to minimize the risk of ulcer formation are necessary lifelong.7
Conclusion
Primary care physicians need to be aware of several key points about the management of obesity. First, obesity is a chronic disease and, as such, requires a lifelong strategy and monitoring for management. Second, optimum treatment is individualized for each patient with respect to social and cultural factors, patient preference, and incorporation with other health care needs.
Third, the treatment plan typically starts with a combination of lifestyle modification, diet, and physical activity. A selected diet should result in a calorie deficit and be sustainable. Ideally, the diet should follow the US Department of Agriculture dietary guidelines by being well-balanced across all food groups to promote both long-term healthy eating habits and a healthy weight. Any level of physical activity has health benefits, but the degree needed for weight loss and maintenance varies considerably for an individual patient.
Pharmacotherapy should be considered as an addition to diet, lifestyle, and exercise when nonpharmacologic treatments alone are unsuccessful and the BMI is 30 kg/m2 or more or 27 kg/m2 or more with obesity-related complications.3,7,17,20 The efficacies of available FDA-approved medications vary. The choice of agent is often made by considering efficacy with cost, patient preference, and specific safety issues or potential for adverse effects.16 In most cases, medications will need to be long-term therapy rather than short-term courses.
Lastly, bariatric surgery is safe and the most successful of treatment options. It becomes a choice for people with a BMI of 40 kg/m2 or more or 35 kg/m2 or more with obesity-related complications.7,11,12,17
1. American Medical Association. Recognition of obesity as a disease H-440.842. AMA Policy Finder. Updated 2013. Accessed May 16, 2022. https://policysearch.ama-assn.org/policyfinder/detail/obesity?uri=%2FAMADoc%2FHOD.xml-0-3858.xml
2. Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clin North Am. 2018;102(1):13-33. https://doi.org/10.1016/j.mcna.2017.08.004
3. Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33(9):1398-1405. https://doi.org/10.1111/liv.12226
4. Obesity and overweight. World Health Organization. June 9, 2021. Accessed May 16, 2022. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
5. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8. https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf
6. Xu H, Cupples LA, Stokes A, Liu CT. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Netw Open. 2018;1(7):e184587. https://doi.org/10.1001/jamanetworkopen.2018.4587
7. Tsai AG, Bessesen DH. Obesity. Ann Intern Med. 2019;170(5):ITC33-ITC48. https://doi.org/10.7326/AITC201903050
8. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 suppl):S176-S185. https://www.ajmc.com/view/obesity-definition-comorbidities-causes-burden
9. Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219-230. https://doi.org/10.1016/j.jhealeco.2011.10.003
10. Dobbs R, Sawers C, Thompson F, et al. Overcoming obesity: an initial economic analysis. McKinsey Global Institute. November 2014. Accessed May 12, 2022. https://www.mckinsey.com/~/media/mckinsey/business%20functions/economic%20studies%20temp/our%20insights/how%20the%20world%20could%20better%20fight%20obesity/mgi_overcoming_obesity_full_report.ashx
11. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. https://doi.org/10.4158/EP161365.GL
12. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023. https://doi.org/10.1016/j.jacc.2013.11.004
13. Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. 2018;21(5):360-365. https://doi.org/10.1097/MCO.0000000000000485
14. Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177-189. https://doi.org/10.1038/s41574-019-0310-7
15. Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the National ACTION Study. Obesity (Silver Spring). 2018;26(1):61-69. https://doi.org/10.1002/oby.22054
16. Bray GA, Ryan DH. Evidence-based weight loss interventions: individualized treatment options to maximize patient outcomes. Diabetes Obes Metab. 2021;23(suppl 1):50-62. https://doi.org/10.1111/dom.14200
17. Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. 2018;102(1):49-63. https://doi.org/10.1016/j.mcna.2017.08.006
18. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. NIH publication number 00-4084. National Institutes of Health, National Heart, Lung, and Blood Institute, North American Association for the Study of Obesity; 2000. https://www.nhlbi.nih.gov/files/docs/guidelines/prctgd_c.pdf
19. Weight loss to prevent obesity-related morbidity and mortality in adults: behavioral interventions. US Preventive Services Task Force. September 18, 2018. Accessed May 16, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/obesity-in-adults-interventions
20. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. https://doi.org/10.1210/jc.2014-3415
21. Wing RR, Look AHEAD Research Group. Does lifestyle intervention improve health of adults with overweight/obesity and type 2 diabetes? Findings from the Look AHEAD Randomized Trial. Obesity (Silver Spring). 2021;29(8):1246-1258. https://doi.org/10.1002/oby.23158
22. Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015;162(7):501-512. https://doi.org/10.7326/M14-2238
23. Healthy weight, nutrition, and physical activity. Centers for Disease Control and Prevention. Reviewed June 3, 2022. Accessed May 12, 2022. https://www.cdc.gov/healthyweight/index.html
24. Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923-933. https://doi.org/10.1001/jama.2014.10397
25. Warrier G, Incze MA. I want to lose weight: which diet is best? JAMA Intern Med. 2021;181(9):1268. https://doi.org/10.1001/jamainternmed.2021.3342
26. MyPlate plan. US Department of Agriculture. Accessed May 16, 2022. https://www.myplate.gov/myplate-plan
27. Phillips JA. Dietary guidelines for Americans, 2020-2025. Workplace Health Saf. 2021;69(8):395. doi:10.1177/21650799211026980
28. Bellicha A, van Baak MA, Battista F, et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: an overview of 12 systematic reviews and 149 studies. Obes Rev. 2021;22(suppl 4):e13256. https://doi.org/10.1111/obr.13256
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. American Heart Association recommendations for physical activity in adults and kids. American Heart Association. Updated April 18, 2018. Accessed May 12, 2022. https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults
31. Iwamoto S, Saxon D, Tsai A, et al. Effects of education and experience on primary care providers' perspectives of obesity treatments during a pragmatic trial. Obesity (Silver Spring). 2018;26(10):1532-1538. https://doi.org/10.1002/oby.22223
32. Modesto-Lowe V, Wick A, Dang M. Physician resistance to obesity pharmacotherapy [letter to the editor]. Cleve Clin J Med. 2021;88(12):658. https://doi.org/10.3949/ccjm.88c.12003
33. Granara B, Laurent J. Provider attitudes and practice patterns of obesity management with pharmacotherapy. J Am Assoc Nurse Pract. 2017;29(9):543-550. https://doi.org/10.1002/2327-6924.12481
34. Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315(22):2424-2434. https://doi.org/10.1001/jama.2016.7602
35. Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi:10.1001/jama.2021.1831
36. Aaseth J, Ellefsen S, Alehagen U, Sundfør TM, Alexander J. Diets and drugs for weight loss and health in obesity—an update. Biomed Pharmacother. 2021;140:111789. https://doi.org/10.1016/j.biopha.2021.111789
37. Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947-1956. https://doi.org/10.1016/S0140-6736(16)00271-3
38. Rosa-Gonçalves P, Majerowicz D. Pharmacotherapy of obesity: limits and perspectives. Am J Cardiovasc Drugs. 2019;19(4):349-364. https://doi.org/10.1007/s40256-019-00328-6
39. Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14(1):12-24. https://doi.org/10.1038/nrendo.2017.122
40. Narayanaswami V, Dwoskin LP. Obesity: current and potential pharmacotherapeutics and targets. Pharmacol Ther. 2017;170:116-147. https://doi.org/10.1016/j.pharmthera.2016.10.015
41. Qsymia (phentermine and topiramate extended-release). Prescribing Information. Vivus LLC; March 2022. Accessed May 18, 2022. https://qsymia.com/patient/include/media/pdf/prescribing-information.pdf
42. Bersoux S, Byun TH, Chaliki SS, Poole KG. Pharmacotherapy for obesity: what you need to know. Cleve Clin J Med. 2017;84(12):951-958. https://doi.org/10.3949/ccjm.84a.16094
43. Tak YJ, Lee SY. Long-term efficacy and safety of anti-obesity treatment: where do we stand? Curr Obes Rep. 2021;10(1):14-30. https://doi.org/10.1007/s13679-020-00422-w
44. Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity: pathophysiology and management. J Am Coll Cardiol. 2018;71(1):69-84. https://doi.org/10.1016/j.jacc.2017.11.011
45. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776-785. https://doi.org/10.1016/S2213-8587(19)30249-9
46. Giruzzi N. Plenity (oral superabsorbent hydrogel). Clin Diabetes. 2020;38(3):313-314. https://doi.org/10.2337/cd20-0032
47. Greenway FL, Aronne LJ, Raben A, et al. A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemic oral hydrogel for weight loss. Obesity (Silver Spring). 2019;27(2):205-216. https://doi.org/10.1002/oby.22347
48. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741-752. https://doi.org/10.1056/NEJMoa066254
49. Kim JH, Wolfe B. Bariatric/metabolic surgery: short- and long-term safety. Curr Atheroscler Rep. 2012;14(6):597-605. https://doi.org/10.1007/s11883-012-0287-3


